Hi everyone!
Here are some updates and reminders for this week:
Chapter 2 and 3 Topic Assignment
The chapter 2 and 3 topic assignment has been graded and grades posted in D2L. I do have one comment about a common error on the quiz.
Question 2 asked for the number of protons, neutrons, and electrons in an iron-58 atom. Several of you answered 26 protons (correct), 32 neutrons (correct) and 28 electrons (incorrect). Some of you explained that it was Fe2-. An Fe atom is neutral (zero charge) by definition. Only an ion has a charge. In a neutral atom, the number of protons and electrons are the same, so the correct number of electrons is 26. So why was 28 electrons a common (incorrect) answer? I suspect it was a result of a Google search like this: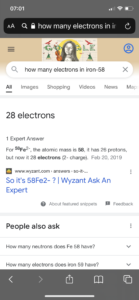
This definitely looks like the likely source of the error. I’m not going to tell you not to google to try to find chemistry help or answers — for better and worse it’s how we get a lot of our information these days, and you probably wouldn’t listen to me anyway :). I will point out some problems/cautions with this approach:
- No doubt google searches can be helpful, but you have to dig a little deeper, read the links the google search gives you and consider the question you are being asked as well as the source writing it. Clicking on the link or reading below said there were 28 electrons in Fe2-. The question wasn’t about an Fe2- ion, it was about an Fe atom.
- Google searches do not always save you time. If you understood that in a neutral atom the number of protons equals the number of electrons and that the atomic number is the number of protons, a quick look at a periodic table would tell you that an iron atom has 26 protons (element 26) and therefore 26 electrons, This would have been faster than a Google search.
It will be helpful for you to think about this as you choose how much to incorporate (or not) google searches into your studies (in addition to looking through the provided material) as you progress through the course.
Also, several of you simply wrote answers for some of the problems on the chapter 2 and 3 quiz without showing work. If you supplied the correct answer without showing any work, you were not awarded credit. Some of the questions that could be answered immediately by inspection (for example, how many protons are in an Fe-58 atom) do not require work to be shown, but calculations do. If you have questions on what “showing work” means, please contact me.
Unit 1 Exam
The Unit 1 Exams are graded. The average was 76.2. You can see your exams and feedback as follows:
If you have questions about any of the exam questions or would like me to go over any of it with you (I’ve already done that with several of you), please contact me and we will schedule a time to go over it.
Showing work on questions 16-20 for exams
A number of you did not turn in your shown work for questions 16-20 on the unit 1 exam. If you supplied the correct answer without showing any work, I awarded you half credit for the problem for this assignments. I did that since in an online course it is easy to forget early in the semester. At this point, you should remember. On future assignments, zero credit will be given for problems if work is not shown, even if the answer is correct. If you have questions on what “showing work” means, please contact me.
Reminders for this week:
- The MyLabsPlus Chapter 4 Homework (Gases)is due Sunday 9/26 at 11:59 pm.
- The Ch 4 Topic Assignment is also due Sunday 9/26 at 11:59 pm.
Please feel free to contact me if you have questions or difficulties.