Objective 1: List some general properties of acids and bases.
We tend to think of acids and bases in the lab, but we encounter them in many places in regular everyday life as well. Examples of acids include vinegar (acetic acid), vitamin C (ascorbic acid), lemon juice (citric acid). Examples of bases include lye (NaOH), ammonia, and baking soda (NaHCO3).
In our study of acids and bases, we will encounter many instances in which they seem complementary or like “opposites”. For example, acids cause blue litmus paper indicator to turn red while bases cause red litmus paper indicator to turn blue. Acids react with bases in neutralization reactions to form water and salts while bases react with acids in neutralization reactions to form water and salts. We’re not really going to do a lot with this objective, but just try to notice this complementary or “opposite” pattern as it repeats through this topic.
Objective 2: Given the chemical formula of any acid or base in the “Formula and Nomenclature” handout, give the name and vice versa.
In order to effectively study acid-base chemistry, it is necessary to be able to identify acids or bases by name or chemical formula. The nomenclature of acids is covered in Sections IV and V of the Formula and Nomenclature Handout on D2L. These sections of the handout also give you many examples of chemical formulas of acids.
You should also recognize the names and formulas of the following bases:
- LiOH (lithium hydroxide)
- NaOH (sodium hydroxide)
- KOH (potassium hydroxide)
- NH4OH (ammonium hydroxide)
- Ca(OH)2 (calcium hydroxide)
- Ba(OH)2 (barium hydroxide)
- Sr(OH)2 (strontium hydroxide)
Identifying acids and bases by chemical formula
Acids are often ionic compounds with H+ as the cation and a nonmetal or polyatomic anion. Formulas for many acids can be found in Table D.1 on this periodic table document in the Unit 4 folder on D2L and in Appendix H of OpenStax.
Examples of bases include the metal hydroxide salts listed above. Another important class of bases include ammonia and the amines.
The Lewis dot structure of ammonia is:
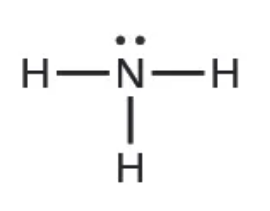
As we will see later, the lone pair on the nitrogen allows ammonia to function as a base.
Amines are organic compounds related to ammonia. For example, methylamine has a similar structure to ammonia, except a carbon bonded to three hydrogens has replaced one of the hydrogen atoms on ammonia:
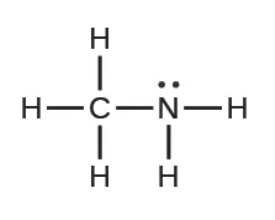
With the the lone pair on the nitrogen, it still functions as a base. Other amines and weak bases are listed by formula in in Table D.2 on this periodic table document in the Unit 4 folder on D2L and in Appendix I of OpenStax.
Objective 3: Given an acid/base reaction identify the Bronsted-Lowry, Arrhenius and Lewis acids or bases present.
Acids and bases are defined three different ways. Each of the three are named after the scientists that proposed the definitions.
Arrhenius acids and bases
Arrhenius acids produce hydrogen (H+) or hydronium (H3O+) ions in water . Arrhenius bases produce hydroxide (OH–) ions in water.
The Arrenhius definitions are limited to acids and bases in water. A hydrogen ion in water — H+(aq) and a hydronium ion — H3O+(aq) are actually two different ways of describing the same thing.
If you have H+ ions (which are essentially just a proton) in water, the H+ ion will be attracted to one of the lone pairs on water.
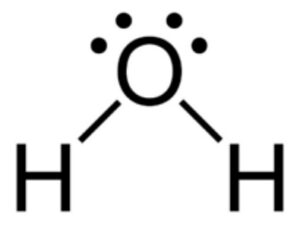
The water will share a lone pair, and the shared lone pair is a single bond, resulting in three hydrogens now being bonded to the central oxygen, or H3O+(aq). This link in LibreTexts provides a good description of hydronium and its equivalence to H+(aq). We will consider H+ (aq) and H3O+ to be interchangeable in this unit, and you are free to write it either way.
As stated above, Arrhenius bases produce hydroxide (OH–) ions in water.
Bronsted Lowry acids and bases
Bronsted-Lowry acids are proton (H+) donors. We can say that a proton and H+ are the same. A hydrogen atom has 1 proton in its nucleus and one electron. Almost all hydrogen is H-1, meaning no neutrons in its nucleus. Therefore, when the H atom loses its electron to form H+, only the proton is left. Bronsted-Lowry acids donate or give up H+ in a chemical reaction. Bronsted-Lowry bases are proton (H+) acceptors. They accept or gain H+ in a chemical reaction.
Lewis acids and bases
The same Lewis that Lewis dot structures are named for proposed this definition. Lewis acids are electron pair acceptors. They form a bond with a lone pair from a Lewis Base. Lewis bases are electron pair donors. They “donate” or share one of their lone pairs with a Lewis acid, forming a chemical bond.
Objective 1 Examples
Hydrofluoric acid (HF) reacts as an acid with water. Let’s look at it with the three theories.
We can write the reaction describing the behavior of HF in water in one of two ways:
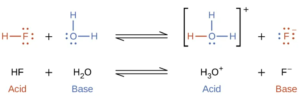
In the top two above, the reaction is written as a reaction of the acid with water to produce hydronium. The first is written in Lewis dot structures and the second using chemical formulas.
In the bottom, the reaction is written as a simple dissociation into hydrogen ion and an anion.
These reactions are equivalent since, in the bottom reaction, the water is there (aq) and since H+ (aq) is equivalent to H3O+.
Arrhenius
HF is an Arrhenius acid. In the equations above, it produces hydrogen (H+) or hydronium (H3O+) ions in water.
Bronsted-Lowry
HF reacts as an Bronsted-Lowry acid with water. HF donates H+to water, so F– is left behind (HF loses one hydrogen and lowers by one unit of charge). Since water accepts H+from HF, becoming H3O+ (water gains one hydrogen and increases by one unit of charge), water is a Bronsted-Lowry base.
Lewis
The H+ from the acid bonds to a lone pair of electrons on the water. This makes the acid a Lewis acid. Water is a Lewis base since it donates its lone pair to form the bond, producing H3O+ .
Ammonia (NH3) reacts as a base with water. Let’s look at it with the three theories.
Arrhenius
NH3 is an Arrhenius base. In the equations above, it produces hydroxide (OH–) ions in water.
Bronsted-Lowry
Ammonia (NH3) reacts as an Bronsted-Lowry base with water.
NH3 accepts H+from water, becoming NH4+(ammonia gains one hydrogen and increases by one unit of charge). Since water donates H+ to NH3 , becoming OH- (water loses one hydrogen and lowers by one unit of charge), water is a Bronsted-Lowry acid.
Lewis
The H+ from the water bonds to a lone pair of electrons on the ammonia. This makes the water a Lewis acid. Ammonia is a Lewis base since it donates its lone pair to form the bond, producing NH4+ .
Relationship between the three definitions
The Arrhenius definition is the most restrictive of the three – it applies only to acidic and basic substances in water. Lewis is the most general or least restrictive – it applies anytime there is a lone pair that forms a bond (it doesn’t even have to involve H+). Bronsted-Lowry is in between. As a result:
- Any Arrhenius acid or base is also a Bronsted-Lowry and Lewis acid or base.
- All Bronsted-Lowry acids and Bases are also Lewis acids or bases, but they may or may not be Arrhenius acids or bases
- Not all Lewis acids or bases are acids or bases under the other two definitions.
Objective 6e: Write simple acid base reactions including the following types:Lewis acid base reactions
Next, we will take a closer look at Lewis acids and bases and their reactions, since for many students it is the toughest one to identify. When a Lewis base gives electrons from lone pair to a Lewis acid, a covalent bond forms between the molecules. The lone pair is shared and becomes the bond.
Examples of Lewis acids:
- Arrhenius or Bronsted/Lowry acids. Remember, any Arrhenius acid or base is also a Bronsted-Lowry and Lewis acid or base.
- Metal cations like Ag+, Fe3+, Cu2+, etc.
- Molecules possessing an atom with less than octet of electrons. BF3 is an example — we will see that below.
- All molecules in which the central atom can exceed an octet of electrons SF4 is an example — it can donate the lone pairs on the central sulfur atom
- Many organic molecules with double or triple bonds. This is not discussed in CHEM 151, but if you go on to take organic chemistry you will see many instances of this.
Examples of Lewis Bases:
- All anions are Lewis bases. OH–, F–, O2- are just a few of many examples.
- All molecules with an atom having lone pairs of electrons.
Example of Lewis acid-base reaction
In the reaction below (shown in OpenStax Section 15.2 – there are other examples of Lewis acid-base reactions shown there as well)
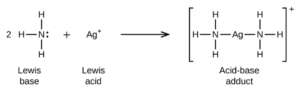
Each ammonia has a lone electron pair that it donates to form a bond with the silver ion. This makes ammonia the Lewis base. By accepting the lone pairs and bonding with the Lewis base, silver acts as the Lewis acid. Notice that the Lewis definition is the only one that applies to this reaction. H+, H3O+, or OH– is not formed in water so Arrhenius does not apply. No H+ is transferred so Bronsted-Lowry does not apply.
Objective 3 and 6e: Examples
In this reaction, H3O+ is formed in water. The Arrhenius theory applies. HI is the acid, making H2O the base. Since the Arrhenius theory applies, Bronsted -Lowry and Lewis apply as well so HI is acting as the acid and H2O the base under all three theories.
In this reaction, H+, H3O+, or OH– is not formed in water so Arrhenius does not apply. H+ is transferred. The HI loses H+ leaving I- and the NH3 gains H+ becoming NH4+ so Bronsted-Lowry does apply. HI is the acid and NH3 is the base. They are also a Lewis acid and base since, if Bronsted Lowry applies, Lewis does as well.
Objective 3 and 6e: Practice
For each reaction below, indicate which acid base theories apply
For the reaction , answer the following question:
Objective 4: Given an acid or base, write the formula of the conjugate base or acid.
Acid-base reactions, according to the Bronsted-Lowry definition, involve proton (H+) transfer. Each acid has a conjugate base, which is what is left after a proton (H+) is removed from the acid. Each base has a conjugate acid, which is formed when the base accepts or adds a proton (H+).
This is the reaction of the weak acid nitrous acid and water:
When nitrous acid donates a proton to water, the nitrite ion NO2– is left behind. NO2– is the conjugate base of HNO2. When water (acting as a base), accepts a proton, it becomes H3O+. H3O+ is the conjugate acid of water.
If we look at the reverse reaction in the same way, we see that HNO2 is the conjugate acid of NO2–, and that water is the conjugate base of H3O+.
HNO2 and NO2– are a conjugate acid-base pair. If HNO2 is the conjugate acid of NO2–, then NO2– must be the conjugate base of HNO2. It is similar to the instance in which a man and woman are a married couple: if the man is the woman’s husband, then the woman is the man’s wife.
What is the other conjugate acid/base pair in the reaction?
This video explains the concept of conjugate acids and bases in more detail: click here for the video. You can read more about them in the OpenStax text in this section.
When ammonia (a base) reacts with water it gains a proton (gains H+) from water, becoming the conjugate acid NH4+. This is illustrated in the figure below, from OpenStax Section 14.1
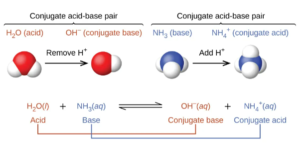
There are many more examples of conjugate acids and bases in that section of OpenStax.
As you may have noticed from the examples, a conjugate acid always has one more hydrogen and one higher (more positive or less negative) charge than its conjugate base.
Objective 4 practice
Objective 6a and 6b:Write simple acid base reactions including the following types:a) dissociation of strong acids, b) ionization of weak acids
Objective 8:Recognize the common strong acids: HCl(aq),HBr(aq),HI(aq),HNO3, H2SO4, HClO4.
Objective 10: Write the equations for the dissociation of weak acids and weak bases and/or Ka and Kb expressions.
Strong acids dissociate completely in water. Hydrochloric acid — HCl (aq) — is an example of a strong acid. As we saw above in objective 1, the reaction can be written as a simple dissociation:
or as an ionization reaction with water:
Since strong acids dissociate or ionize completely (they are strong electrolytes, as we discussed in the Unit 2 topic of reactions and solutions), we write is as a one-way reaction with a single arrow.
Weak acids dissociate partially in water in an equilibrium reaction. As with strong acids, they can be written as a simple dissociation, except with a two-way (equilibrium) double arrow:
or as an ionization reaction with water:
The only way to know whether an acid is strong or weak is to memorize or know the strong acids. They are:
- HCl(aq)
- HBr(aq)
- HI(aq)
- HNO3
- H2SO4
- HClO4.
Other acids are weak.
Ka (acid dissociation constant)
Ka (acid dissociation constant) is the equilibrium constant for the acid dissociation or ionization. The expression for Ka can be written just like any other expression for K as you learned recently in your study of equilibrium.
For the reaction :
For the reaction :
We ignore the water since it is a pure liquid (l). Since H+ and H3O+ are the same, these two expressions for Ka are the same. It is acceptable to write it either way.
Note that Ka has the expression:
This will be true for any weak acid. Ka will always have [H+] (or hydronium) and [the conjugate base of the acid] in the numerator and [the acid] in the denominator for any weak acid.
Given any weak acid, you should be able to write its ionization reaction (either form is OK) and Ka expression.
Weak acids and their numerical Ka values are listed on this periodic table handout also available on D2L and this table of Ka values in OpenStax.
Strong Bases are bases that completely ionize in water to produce hydroxide ions. They are best written as simple dissociations rather than reactions with water.
An example is sodium hydroxide (NaOH):
Strong bases are group I and group II metal hydroxides. You should recognize these eight:
- LiOH (lithium hydroxide)
- NaOH (sodium hydroxide)
- KOH (potassium hydroxide)
- RbOH (rubidium hydroxide)
- CsOH (cesium hydroxide)
- Ca(OH)2 (calcium hydroxide)
- Sr(OH)2 (strontium hydroxide)
- Ba(OH)2 (barium hydroxide)
Weak bases ionize partially (usually only slightly) in water. Like weak acids, they are equilibrium reactions rather than one way or complete. Their equilibrium constants are called Kb or base dissociation constants.. Unlike weak acids, their ionization reactions must be written as ionizations reactions with water – NOT as dissociations. Note in the reactions they accept a proton from water (act as Bronsted-Lowry bases).
Kb (base ionization constant)
Kb (base ionization constant) is the equilibrium constant for the base’s ionization reaction with water. For the weak base ionization above (ammonia or NH3), the expression is:
Note that Kb has the expression:
This will be true for any weak base. Kb will always have [OH–] and [the conjugate acid of the base] in the numerator and [the base] in the denominator for any weak base.
Weak bases and their numerical Kb values are listed on this periodic table handout also available on D2L and this link of Kb values in OpenStax.
How does Ka (or Kb) value connect with acid (or base) strength?
Strong acids dissociate completely. You cannot look up numerical Ka. The Ka is sometimes described as “large” or approaching infinity. For weak acids, the higher the Ka, the more the dissociation equilibrium lies to the right, and the stronger (or less weak) the weak acid.
Bases are similar. Strong bases dissociate completely. You cannot look up numerical Kb. For weak bases, the higher the Kb, the more the ionization equilibrium lies to the right, and the stronger (or less weak) the weak base.
More reading on these objectives is available here.
Objective 6,8,10 practice
The expression represents the
The expression represents the
Objective 7: Describe the autoionization of water and write and apply the Kw expression.
An amphoteric substance an act as either an acid or a base. Amphoteric substances typically have both transferable H and an atom with lone pair. Water is an example of an amphoteric substance. Recall we have seen it act as an acid (donating a proton) in some reactions and a base (accepting a proton) in others.
Other amphoteric substances include NH3 and anions that include a removable hydrogen like HCO3–, HSO4–, and H2PO4–. For example, HCO3– can gain a hydrogen ion to form H2CO3 or lose a hydrogen to form CO32-.
In autoionization, an amphoteric substance undergoes an acid/base reaction with itself. Water undergoes autoionization in this reaction:
(written as a reaction with water)
or (written as a simple dissociation)
We can write an equilibrium expression for the autoionization of water just like we have other equations. It is or
Note water, as a pure liquid (l), is left out. This special equilibrium constant is referred to as the ion-product constant for water, Kw. At 25°C, Kw = 1.0 × 10-14.
Objective 5: Explain the relationship between the strength of an acid and the strength of its conjugate base.
Objective 15: Interconvert between the Ka of an acid and the Kb of its conjugate base
Let’s look at the conjugate acid base pair HF and F–.
The Ka expression for HF is:
The Kb expression for F– is:
Multiplying this Ka and Kb,
This will be true for all conjugate acid base pairs in water. Let’s look at that a little more closely. For a conjugate acid base pair,
KaKb=Kw=10-14
Examples:
This is useful because
- If we know (or can look up) the Ka of a weak acid, we can calculate the Kb of its conjugate base (or vice versa). The conjugate values are usually not tabulated, so this is useful
- The larger the Ka of an acid, the smaller the Kb of its conjugate base (and vice versa). This means the stronger a weak acid is, the weaker its conjugate base is (and vice versa). They are still both weak, though. The conjugate base of a weak acid is still a weak base.
Objective 5 and 15 example
This example uses data from this periodic table handout made available to all CHEM 151 students. It can also be found in the Unit 4 folder of your course D2L site.
Calculate the Kb of the acetate ion, C2H3O2–.
Similarly, the Kb of the chlorite ion, ClO2– can be calculated to be 9.1 x 10-13. Since the Kb of acetate is larger than the Kb of chlorate, acetate ion is the stronger base (note both conjugate bases are still weak, though).
Objective 5 and 15 practice
Objective 6: Calculate [H+], pH and/or [OH–], pOH given the value of any one of the variables or the concentration of a strong acid or base.
In acid-base chemistry, the molarities of acids and bases (and their hydroxide and hydrogen ion molarities) are typically very small. One way we handle those very small numbers is with scientific notation:
Examples
[H+]=0.001 M = 10-3 M
[OH–]=0.00001 M = 10-5 M
Another way we handle these numbers is with p-functions. In a p-function, the negative base-10 logarithm of the molarity is taken:
This results in numbers that are much higher to deal with:
For [H+]=0.001 M = 10-3 M:
For [OH–]=0.00001 M = 10-5 M:
Numbers like 3 and 5 are much nicer to work with, so pH and pOH are commonly used to quantify acidity or basicity.
The following relationships can be used to interconvert between [H+], pH, [OH–], and pOH – given any one of the four, you should be able to calculate the other three.
A good overview of pH and pOH can be found in OpenStax Section 14.2
Equations for [H+], pH, [OH–], and pOH
Objective 6 example
Calculate the [H+], [OH–], and pOH of a pH 5.65 solution
, so
Relative acidity or basicity from pH
Also, note that the lower the pH, the more acidic the solution, and the higher the pH, the more basic the solution. This is illustrated nicely in Table 14.2 of OpenStax.
Let’s take a closer look at a portion of that table:
| [H+], M | [OH–], M | pH | pOH | acidic, basic, or neutral |
| 10-4 | 10-10 | 4 | 10 | acidic |
| 4.0 X 10-5 | 2.5 X 10-10 | 4.40 | 9.60 | acidic |
| 10-5 | 10-9 | 5 | 9 | acidic |
| 10-7 | 10-7 | 7 | 7 | neutral |
| 10-8 | 10-6 | 8 | 6 | basic |
This table tells us a few things about pH
- the first three rows show that if pH <7 that [H+] > [OH–]. Since [H+] is indicative of acidity and [OH–] indicates basicity, solutions are acidic for pH<7.
- the last row shows that if pH >7 that [H+] < [OH–]. Since [H+] is indicative of acidity and [OH–] indicates basicity, solutions are basic for pH>7.
- the fourth row shows that if pH =7 that [H+] = [OH–]. Since [H+] is indicative of acidity and [OH–] indicates basicity, solutions are neutral for pH=7.
- In the second row, [H+] is between 10-4 and 10-5, so pH is between 4 and 5.
- Since pH and pOH are logarithmic values, the number of decimal places are the significant figures. In the pH of 4.40, the 4 before the decimal point results from the 10-5 in the [H+], and the .40 after the decimal point are the two sig figs.
- A pH increase of 1 means 1o times higher concentration of H+
Calculating the pH of Strong Acids or Bases
Strong acids and bases ionize completely. Therefore, for a monoprotic strong acid [H+] = [Acid] and for a monofunctional base [OH–] = [base] (a monofunctional base is a base with one hydroxide). Let’s look at why….
Let’s write an ICE table for the ionization of a 0.1 M solution the strong acid HCl that looks like an ICE table but isn’t exactly an ICE table, since strong acids dissociate completely:
| HCl | ↔ | H+ | Cl– | |
| I | 0.1 | 0 | 0 | |
| C | -0.1 (complete dissociation) | +0.1 | +0.1 | |
| “E” | 0 | 0.1 | 0.1 |
The “equilibrium” concentration if H+ (0.1M) is the same as the initial concentration of the HCl, since all of the 0.1 moles per liter dissociate. This “ICE table” is not needed if we recognize that.
This is not true for weak acids or bases since they do not ionize completely. A real ICE table , with an actual equilibrium, is necessary to determine pH for weak acids or bases.
As discusses earlier, strong bases include Group 1 and 2 metal hydroxides. The list of strong bases is repeated here:
- LiOH (lithium hydroxide)
- NaOH (sodium hydroxide)
- KOH (potassium hydroxide)
- RbOH (rubidium hydroxide)
- CsOH (cesium hydroxide)
- Ca(OH)2 (calcium hydroxide)
- Sr(OH)2 (strontium hydroxide)
- Ba(OH)2 (barium hydroxide)
For bases
Examples
What is the pH of a 0.20 M solution of HCl?
Since HCl is a strong acid, [H+] = 0..20 M and pH = -log(0.20)=0.70
An aqueous solution of HClO4 has a pH of 2.56. What is the concentration of the acid?
If pH = 2.56, , and the acid concentration is also
since it is a strong acid.
Objective 6 practice
Answer the following questions for a pH 3.20 solution:
Answer the following questions for more practice
Objective 14: Use pKa and pKb to predict the relative acid base strengths.
The idea of p-functions also works well with Ka and Kb values, which are also typically small. Again, p means -log so:
and
Also, for a conjugate acid-base pair, since KaKb=Kw it also means
pKa + pKb = 14
Objective 14 Example
For acetic acid (HC2H3O2), . Therefore,
For nitrous acid (HNO2), . Therefore,
This shows that the larger the Ka, the lower the pKa.
Nitrous acid, with its larger Ka and lower pKa value, is a stronger weak acid than acetic acid.
We can also calculate the pKb values of the conjugate bases C2H3O2– and NO2–.
For C2H3O2– : pKb = 14-4.74=9.26
For NO2–: pKb = 14-3.34=10.66
Previously we saw that nitrous acid, with its larger Ka and lower pKa value, is a stronger weak acid than acetic acid. Therefore base its conjugate base nitrite ion is a weaker base than acetate ion, as shown by its higher pKb.
Objective 14 practice
Using the Kb values of ammonia (NH3) and dimethylamine ((CH3)2NH) from Appendix I in OpenStax, answer the following questions:
Objective 11: Calculate all equilibrium concentrations and pH or pOH in a solution of a weak acid or base given the Ka or Kb or vice versa.
Earlier, we saw that in the case of a strong monoprotic acid or a strong base with one hydroxide, the [H+] (for an acid) or the [OH–] (for a base) was equal to the molarity of the strong acid or base. This is not the case for a weak acid or base as they do not ionize completely.
For the nitrous acid equation above, [H+] will be less than the original acid molarity due to the incomplete dissociation. Similarly for the weak base ammonia, the [OH–] will be less than the original base molarity. How do you determine the concentrations (and therefore the pH)? By using an ICE (initial, change, equilibrium) table.
An example of this technique is shown in Example 14.12 of Section 14.3 of OpenStax (scroll down to Example 14.12 after opening the link) or in the video below:
The similar problems for bases are demonstrated in Example 14.13 in the same OpenStax section.
Here is a link to another video example for a pH of a weak acid calculation and a weak base calculation from the same video..
Objective 11 Practice
Objective 12: Calculate the Ka/Kb for a weak acid/base given the pH of a solution of known concentration.
Objective 12 is essentially Objective 11 in reverse – instead of calculating the [H+] and the pH using the given Ka we calculate the [H+] from the given pH then calculate the Ka using the ICE table.
Examples of these calculations are found in Section 14.3 of OpenStax (scroll to examples 14.11 and 14.10). The video below will also illustrate a solution for this example:
The pH of a 0.10 M solution of an unknown weak acid is 2.17. Calculate the Ka of this unknown weak acid.
Objective 12 Practice
Objective 13: Calculate percent ionization of an acid or base from initial concentrations and Ka or Kb values or vice-versa.
Consider a 0.125 M solution of nitrous acid (a weak acid), which has a Ka of 4.6 X 10-4.
The dissociation equation is:
If you were to do an ICE table calculation to determine its pH (see Objective 11), the table would be:
| HNO2 | ↔ | H+ | NO2– | |
| I | 0.125 | 0 | 0 | |
| C | -x | +x | +x | |
| E | 0.125-x | x | x |
Using the expression for Ka and solving for x (see Objective 11 above for how to do this) yields x= 7.5 X 10-3.
Percent dissociation or percent ionization is defined as the percentage of the original acid or base that ionizes. It can be expressed as:
For the above example, .
Given the percent ionization and initial molarity , you should also be able to solve for the x in the ICE table using the equation
.
From there, you could calculate pH. The following practice problems will let you try these calculations.
You can read more about percent dissociation in OpenStax Section 14.3.
Objective 13 Practice
Objective 16: Write the Ka expressions for the stepwise ionization of a polyprotic acid and understand the significance of magnitude differences of Ka1 and Ka2.
Most acids are monoprotic, meaning “one proton” — they can lose one hydrogen. Polyprotic acids are acids that have more than one ionizable hydrogen.
Examples of polyprotic acids include:
- H2SO4 (diprotic)
- H3PO4 (triprotic).
Polyprotic acids ionize in steps, with one step for each hydrogen. This is called stepwise dissociation.
Stepwise dissociation for H2SO3
As an example, let’s look at the weak diprotic acid sulfurous acid (H2SO3).
First dissociation:
Second dissociation:
Each dissociation has an expression and a value for Ka (numbered Ka1, Ka2, etc.)
First dissociation: = 1.6×10-2
Second dissociation: = 6.4×10-8)
Each hydrogen ion gets harder to remove.For example, H2SO3 is a stronger acid than HSO3−. Ka1 is the equilibrium constant for the first dissociation (losing the first hydrogen ion), Ka2 is the equilibrium constant for the second dissociation (losing the second hydrogen ion). For most polyprotic acids, there are three orders of magnitude between successive Ka values (Ka1/Ka2> 103). Thus is the case for sulfurous acid (see Ka values above).
Objective 16 Practice
Objective 17: Using the balanced chemical equations for both the cation and anion, predict whether a salt solution will be acidic, basic or neutral.
Objective 6d: Write simple acid base reactions including the following types: the dissociation of salts in water
Hydrolysis is the reaction of an ion with water. When a salt dissolves in water, it breaks up into a positive cation and a negative anion. One or both of these ions may react with the water, undergoing hydrolysis.
Hydrolysis of cations:
A cation may react with water. If it does, it acts as a Bronsted- Lowry acid, donating a proton to water. So one of two things will happen with the cation in water:
- It does not have a proton to donate to water and therefore will not react (will not undergo hydrolysis). There are typically metal cations. For example: Na+ + H2O → no reaction. These cations will be neutral in water.
- It does have a proton to donate to water and therefore will react (will undergo hydrolysis). There are typically cations that are conjugate acids of weak bases. For example
. These cations will be acidic in water.
Hydrolysis of anions:
A anion may react with water. If it does, it acts as a Bronsted- Lowry base, accepting a proton from water. So one of two things will happen with the anion in water:
- It will not accept proton from water and therefore will not react (will not undergo hydrolysis). These anions are typically anions of strong acids. For example: Cl-1, the anion of the strong acid HCl, does not react with water.
. These anions are neutral in water.
- It does have a proton to donate to water and therefore will react (will undergo hydrolysis). There are typically anions of weak acids. For example:
. These anions are basic in water.
For further reading, see OpenStax Section 14.4. A description is also shown in this video.
Objective 17 Practice
Calculating the pH of salt solutions.
We just saw that in a solution of KF that the K+ cation is neutral and the F– anion is basic, the salt solution will be basic. It is basic due to the hydrolysis reaction: .
Note this is an ionization reaction of the weak conjugate base F–, with the following Kb expression:
We could calculate the Kb value using the tabulated Ka of HF:
Let’s say we wished to calculate the pH of a 0.10 M KF solution. Since KF dissociates completely, the initial F– molarity is 0.10 M. So now you can do a calculation using an ICE table just like those in Objective 11.
| F– | H2O | ↔ | HF | OH– | |
| I | 0.10 | 0 | 0 | ||
| C | -x | +x | +x | ||
| E | 0.10-x | x | x |
Solving for x (which is [OH–]), we get x=[OH–]=1.3 X 10-6, which results in a pH of 8.10.
Example 14.15 in Section 14.4of OpenStax shows an example with an acidic salt. Additional examples are available in this video.
Objective 17 and 6e Practice
Objective 19: Use knowledge of chemical structure to predict relative acid-base strength.
By understanding the molecular structure and bonding of acids, we can use it, combined with a knowledge of periodic trends, to predict relative acid-base strength between similar types of acids (and therefore their conjugate bases). We will look at this for three types of acids in a moment. Before that, let’s examine the connection between bonding and acid strength.
The stronger an acid, the more it dissociates, giving up H+. Strong acids dissociate completely (100%) in water. Weak acids do not, but the higher their percent dissociation, the stronger the weak acid. We can think of all acids as having a hydrogen atom bonded to the rest of the molecule:
H-rest of molecule
The weaker the bond between H and the rest of the molecule, the more easily it is broken, and the higher the tendency for dissociation. Therefore, the weaker the bond between H and the rest of the molecule, the stronger the acid.
Binary Acids:
Binary acids
Binary Acids:
Binary acids are acids made up of hydrogen and one other element. Examples include:
- HF
- HCl
- Hbr
- H2O
- H2S
- and others
In general , we can say they have the formula H-X, where X is the element other than hydrogen. For element X, binary acid strength increases top to bottom down the group as the larger anion results in a weaker H-X bond.
Example: HF, HCl, HBr, HI is listed weakest acid to strongest acid.
Also, for element X, binary acid strength increases from left to the right across a period due to increased polarity of H-X.
Example: CH4 , NH3, H2O, HF is listed weakest acid to strongest acid.
Acids with the same number of oxygen atoms
The more electronegative the atom that is not H or O, the stronger the acid. The more electronegative atom pulls electron density away from the O-H bond, weakening it.
Examples: HClO, HBrO , and HIO is listed strongest to weakest acid, since Cl. Br, and O are listed in order of highest to lowest electronegativity. The same would be true for HClO3 , HBrO3 , and HIO3
Acids containing oxygen that differ only in the number of oxygen atoms
For a series of oxyacids, acidity increases with the number of oxygens. The presence of more highly electronegative oxygen atoms weakens the O-H bond.
Examples:
- HNO3 is a stronger acid than HNO2 .
- HClO4 , HClO3 , HClO2 , HClO are listed strongest to weakest acid.
Relative conjugate base strength
Remember the weaker an acid, the stronger its conjugate base. We just saw that HClO4 , HClO3 , HClO2 , HClO are listed strongest to weakest acid. Therefore we could list their conjugate bases as well:
ClO4– ,ClO3– ,ClO2– , ClO– are listed weakest base to strongest base.
Objective 19 practice
Objective 6c: Write simple acid base reactions including the following types: the addition of strong acid/base to weak base/acid
These reactions are acid-base neutralization reactions, which were covered in Unit 2. The following is reprinted from the Bruce’s notes on Aqueous Reactions and Solution Stoichiometry, and will allow you to review this topic:
Neutralization Reactions
Acid base neutralization reactions are, like the precipitation reactions we have seen, double replacement reactions. An example is:
Typically we do not write the combination of the hydrogen ion from the acid and the hydroxide ion from the bases as “HOH”, though, we instead write it as:
These reactions have the general form:
acid + base →salt + water.
Like the precipitation reactions we have seen, you can also write molecular, ionic, and net ionic equations for these.
Go to the Chemical Reactions Handout on the course D2L site. To find it click “content”, then click “Reaction Handout CHEM 151”, then click “CHEM 151 Reaction Handout”. Neutralization reactions are discussed in detail along with precipitation equations in Section 6 of the Handout beginning on page 33 of the handout. Included are step by step examples on how to identify, predict and write these equations, a section on common errors, and practice questions with answers.