Before beginning with the objectives, let’s start with some definitions that will be used throughout this topic:
Some Definitions for Solution Chemistry
Solution – A solution is a homogeneous mixture of two or more pure substances. A solution consists of a solvent and one or more solutes.
Solvent – The solvent is the substance that dissolves the solute. In an aqueous solution, water is the solvent. Water is the most common solvent we will encounter, and most of our work will be with aqueous solutions.
Solute – A solute is a substance that is dissolved into the solvent.
Solubility – how much of the solute dissolves in the solvent. We will look at solubility qualitatively (without numbers) in this class – whether a solute will dissolve, will not dissolve, will dissolve completely, or just a little bit. In CHEM 152, solubility is studied quantitively — for example, by calculating how many grams of a solute can dissolve in a liter of solution.
Normally, it is straightforward to identify the solvent. If a substance changes phase to enter the solution, it is generally a solute. The solvent generally keeps its phase. For example, when a solid salt dissolves into liquid water to make salt water (which acts as a liquid solution) the water is the solvent. For solutions that are all the same phase, the solvent is the substance that is the greatest amount. For example, air is a solution made up of gases. Its composition is:
- 78% nitrogen
- 21% oxygen
- 1% other, including carbon dioxide, neon, hydrogen, and more
Nitrogen is the solvent in air. Oxygen, and all the other gases, are solutes.
Objective 13: Given the chemical formula of any atom, ion, or compound listed in the “Formula and Nomenclature” handout, give the name and vice versa.
The Formula and Nomenclature handout on D2L tests you with questions like “given the name write the formula” and “given the formula write the name (and that is what the handout asks you to do). While that is good practice, that is not the real value of the handout or the reason for understanding formulas and names. As you will see in this chapter, knowing the difference between molecular and ionic compounds, knowing the charges on the single atom and polyatomic ions, and understanding how (and in what ratios) ionic compounds combine and break apart is essential for success in this unit.
Continued work on the formula and nomenclature handout (especially if most of it was new to you or you had forgotten a lot of it since you previously studied chemistry) will make the rest of this course (and especially this chapter) much easier.
Objective 1: Write equations to show ion formation from electrolytes when they ionize or dissociate. (See Chemical Reaction Handout)
This diagram exhibits on an atomic level what happens when a salt dissolves in water:
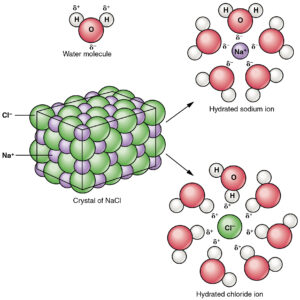
| Anatomy & Physiology, Connexions Web site. http://cnx.org/contents/e4e45509-bfc0-4aee-b73e-17b7582bf7e1@4, Jun 19, 2013. https://creativecommons.org/licenses/by/3.0/deed.en |
We can represent that process with a dissociation equation or ionization equation:
The solid NaCl crystal is broken apart and dissolved by the water, as water molecules pull apart and surround the individual Na+ and Cl–ions. Ionization means the products will be ions (and have charges).
Notice this is different from the decomposition reactions you learned about in the last unit – the decomposition of NaCl is .
These are very different reactions!
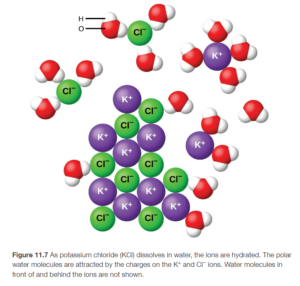
OpenStax Figure 11.7 shows a similar process with KCl:
Examples – dissociation or ionization equations
Example 1: Write the balanced equation that occurs when K2SO4 is dissolved in water.
First, write a reaction in which the reactant salt dissociates into its ions (make sure the ions have the proper charges):
Notice that you must know potassium ion has a 1+ charge and sulfate (SO42-) has a 2- charge to correctly write this equation. Notice even though there are 2 potassiums it does not dissociate into K2+ — we will take care of balancing it next.
Next, balance the equation:
A coefficient of 2 is necessary to balance potassium and to balance charges for the reaction.
Another example is shown in this video.
Practice – dissociation or ionization equations
Objective 2: Define and identify substances as strong, weak and non electrolytes.
Read Section 11.2 in OpenStax. It has a nice overview of the material on electrolytes discussed next.
Electrolytes
Electrolytes are substances that produce ions when dissolved in water. Therefore, their aqueous solutions contain ions and better conduct electricity. Ionic compounds and acids are examples of electrolytes. We saw the dissociation of NaCl in the previous section:
It is an electrolyte as it produces ions when it dissolves in water. Electrolytes can be further divided into strong electrolytes and weak electrolytes.
Strong Electrolytes
Strong electrolytes dissociate completely or virtually completely when dissolved in water. They exist in aqueous solution completely or almost completely as ions. Their aqueous solutions conduct electricity due to the abundance of ions.
Dissociation reactions for strong electrolytes are written as one-way reactions with a single arrow →, indicating they happen completely.
Examples of strong electrolytes
Soluble salts and marginally soluble salts. Salts are ionic compounds from Sections 2 and 3 of the Formula and Nomenclature handout on D2L. We can tell whether they are soluble using solubility rules, which will be discussed in Objective 3.
Strong acids are acids which are strong electrolytes. An example is hydrochloric acid:
Strong bases are bases which are strong electrolytes. Bases produce hydroxide ion (OH–) in aqueous solution:
Weak Electrolytes
Weak electrolytes only dissociate partially when dissolved in water. They exist in solution mostly in the form of molecules with only small fractions in the form of ions. Their solutions conduct electricity less efficiently than strong electrolytes since there are fewer ions in solution.
Dissociation reactions for weak electrolytes are written as two-way reactions with a double arrow , indicating they are in equilibrium with reactant and product co-existing.
AgCl stays mostly intact in water, with only a very small fraction dissociating to form ions, making it a weak electrolyte.
Examples of weak electrolytes
Insoluble salts. Even insoluble ionic compounds dissolve and produce ions, just not enough to be meaningful. We can tell whether salts are insoluble using solubility rules, which will be discussed in Objective 3.
Weak acids are acids which are weak electrolytes. An example is acetic acid:
Weak bases are bases which are weak electrolytes.
Nonelectrolytes
Nonelectrolytes are substance that do not produce ions when dissolved in water. Their aqueous solutions do not contain any ions, and therefore do not conduct electricity well. Nonelectrolytes do NOT dissociate via ionization equations discussed in objective 1, even though they dissolve.
Most nonelectrolytes are molecular compounds (made up of all nonmetals). The molecules stay intact in solution even when the nonelectrolyte dissolves. With the exception of the acids, compounds that are made up of all nonmetals are nonelectrolytes. Examples of nonelectrolytes are alcohols and sugars. Alcohols generally have the formulas CxHyOH. Sugars generally have the formulas CxHyOz.
Objective 3: Use solubility rules to predict the water solubility of salts.
As discussed earlier, solubility is a measure of how much solute dissolves in a solvent. We will look at solubility qualitatively in CHEM 151 — that is whether it dissolves well (soluble) or does not dissolve — or dissolves very little (insoluble). Patterns have been observed for ionic compounds (salts) over many experiments. These patterns are summarized in solubility rules, and are used to tell whether or not salts are soluble (strong electrolytes) or insoluble (weak electrolytes).
There are many, many different sets of solubility rules published in textbooks, reference books, and online. The reasons for this is that most of them are partial lists of rules, and they are organized in different ways. Examples include the one in Chapter 4 of the Brown and LeMay text and Table 4.1 in OpenStax. If you are asked questions from a particular source, it is best to use the list of rules presented from that source (for example, use the list suggested by MyLabsPlus for MyLabsPlus homework). For CHEM 151 quizzes or exams, use this set:
Solubility Guidelines for Common Ions in Water for CHEM 151
- All compounds of the ammonium ion (NH4+), and of alkali metal (Group IA) cations, are soluble
- All nitrates (NO3–) and acetates (CH3COO– or C2H3O2–) are soluble
- All Cl–, Br–, and l– salts are soluble EXCEPT those of Ag+, Pb2+ and Hg22+
- All SO42- are soluble EXCEPT those of Ba2+, Sr2+, Ag+, Hg22+,and Pb2+
- All OH– salts are water insoluble except group I and ammonium. Ba(OH)2, Sr(OH)2, and Ca(OH)2 are marginally soluble.
- All S2-, and CO32- are insoluble EXCEPT those of NH4+ and Alkali metal (Group IA) cations
Remember, when applying the solubility rules
- Soluble or marginally soluble salts are considered strong electrolytes
- Slightly soluble and insoluble salts are considered weak electrolytes
- Solubility rules are only used for salts. Do not use solubility rules to determine whether acids or bases are weak or strong.
Solubility Rule Examples
The salt K2SO4 is soluble by rule 1 or rule 4 (two rules are not needed — one is enough)
The salt SrSO4 is insoluble by rule 4 (one of the exceptions)
The salt Pb(NO3)4 is soluble (rule 2)
The salt Pb(OH)2 is insoluble (rule 5)
Solubility Rule Practice
Objective 4: Knowing the solubility rules, predict whether a precipitation reaction will occur.
Precipitation reactions are reactions between aqueous solutions of ionic compounds that produce an ionic compound that is insoluble in water. The insoluble product (solid) is called a precipitate. To predict whether certain combinations of ions form insoluble products (solids precipitates), we use the solubility rules.
Precipitation reaction example
Mixing Pb(NO3)2 and KI, both of which are soluble, produces an insoluble solid. Take a look at Figure 4.4 in OpenStax – it shows the yellow precipitate that is formed. Also look at the chemical equations just above the figure — these show the reactions that take place.
Let’s think about what the yellow precipitate might be. The Pb(NO3)2 will dissociate to form Pb2+ and NO3– ions. The KI will dissociate to form K+ and I– ions. That means the following ions will be in solution: Pb2+ , NO3– , K+ , and I– . If any two of these can combine to make a neutral, insoluble product, there will be a precipitate. Pb2+ and I– ions will. To make a neutral product, there will be two iodide ions for every one lead ion, so the precipitate will be PbI2, which is insoluble (solubility rule 3). This is also shown in this video.
Objective 4 practice
Objective 5: Write balanced molecular, complete ionic and/or net ionic equations.
Three types of chemical equations are used to represent precipitate reactions: molecular, ionic, and net ionic equations. The molecular equation gives the complete formulas for all species as though they existed as molecules in solution.
The complete ionic equation shows the individual ions present in the solution. Insoluble salts and molecular compounds remain as one unit (do not dissociate), strong electrolytes written as ions.
The net ionic equation shows only species that change during a chemical reaction and eliminate spectator ions which appear in the same form on both sides of the reaction.
The procedure for writing these three types of equations is given in detail in the Chemical Reactions Handout which is located in the Reaction Handout folder in the course D2L site (see Reaction Handout CHEM 151 in the Table of Contents). A specific example is shown step by step as Example 6a on page 34 and 35 of the handout. Useful background information is given on page 36 under section 6: Double Replacement Reactions on page 33.
This video illustrates step-by-step how to write these equations.
Objective 6: Differentiate between the terms acid and base, strong acid and weak acid.
Objective 7: Identify the common strong acids and bases.
Acids
Acids are substances that ionize in aqueous solutions to form hydrogen ion. An example is the reaction
Strong Acids Strong acids are acids that are strong electrolytes, which mean they completely dissociate into anion and H+ in water. There are virtually no intact molecules in their solutions. We do not use solubility rules to determine which acids are strong acids. Unlike salts, acids can be water soluble but still be weak. The only way to know which acids are the strong ones are to memorize the seven strong acids.
List of strong acids
- Hydrochloric acid (HCl)
- Hydrobromic acid (HBr)
- Hydroiodic acid (HI)
- Nitric acid (HNO3)
- Sulfuric acid (H2SO4)
- Chloric acid (HClO3)
- Perchloric acid (HClO4)
Weak acids are acids that are weak electrolytes. They ionize slightly in water (shown by equilibrium reactions) All acids not on the list of the seven common strong acids above are considered weak. An example of a weak acid is acetic acid:
Bases
Bases are substances that produce OH− ions when dissolved in water.Strong bases are bases which are strong electrolytes. They dissociate completely in water. The strong bases are certain hydroxide salts:
List of strong bases
- Group 1A metal hydroxides (LiOH, NaOH, KOH, RbOH and CsOH)
- heavy group 2A hydroxides, Ca(OH)2, Sr(OH)2 and Ba(OH)2
An example of a dissociation reaction is:
Weak bases are bases which are weak electrolytes. They are primarily compounds containing N and H or C, H and N (examples include NH3, C2H5NH2). Weak bases provide hydroxide by removing a hydrogen ion from water:
We will study weak bases in more detail in Unit 5.
Objective 9: Calculate molarity, solution volume, or number of moles of solute, given any two of these quantities.
Concentration is a measure of how much solute is dissolved in an amount of solution. We say the the solution is concentrated if the concentration is high or dilute if the concentration is low. There are many ways to express concentration quantitatively or numerically. The most common one is molarity. We will work with molarity in this unit, and later in the semester we will work with other concentration units as well.
Molarity
Molarity is defined as the moles of solute per liter of solution:
An upper case M is used to signify molarity, so when writing units M is equivalent to mol/L, and the use of either is correct. For example,
0.50 M Na2SO4 is the same as .
In English, we would read it as “0.50 molar sodium sulfate”. Note the M means mol/L. Students are often tempted to abbreviate moles by using M or m – neither are correct. If you wish to shorten “moles” when expressing units, use “mol”.
In the molarity equation above, there are three variables: M, moles of solute, and volume of solution. Given any two, you should be able to solve for the third (just as you did with density in unit 1). You can use either an algebraic approach by plugging given values into the equation and solving, ot you can use dimensional analysis and use mol/L as a conversion factor. Let’s look at an example:
Objective 9 Example (Molarity)
Calculate the mass in grams of KNO3 needed to make 50.0 mL of a 0.50 M KNO3 solution.
Looking at the equation: , we are given volume (in mL, but it can be converted to L) and molarity. Given those we can solve for moles (which can be converted to mass in grams).
We can algebraically rearrange
to
Plugging in the given values (and converting 50.0 mL to 0.0500L),
Converting to grams,
We could use dimensional analysis as an alternative. Realizing we are given mL, we have a conversion factor of mol/L (remember molarity is mol/L), and we want mass in grams:
.
If you choose to work in dimensional analysis, always use mol/L instead of M for molarity. You should never use M in dimensional analysis – the units will not cancel properly and it will lead to confusion.
Objective 9 Practice (Molarity)
Objective 10: Calculate the concentration of ions present for the solutions of strong electrolytes.
Let’s consider a 0.50 M solution of the strong electrolyte Na2SO4. As a sodium (Group I) salt, it is a strong electrolyte. Its dissociation equation is:
As a strong electrolyte, it will dissociate completely, leaving no intact Na2SO4 in solution.
Since every one mole of Na2SO4 provides 2 moles of Na+ ions, the concentration of sodium ions is double that of the Na2SO4, or 1.0 M
Since every one mole of Na2SO4 provides one mole of SO42- ions, the concentration of sodium ions is the same as that of the Na2SO4, or 0.50 M.
Therefore, a 0.50 Na2SO4 solution will be 1.0 M in Na+ and 0.50 M in SO42-.
Objective 10 practice
Objective 11: Solve problems involving dilution of solutions.
When you hear the word dilution, it is most likely that you think of adding water. Strictly speaking, dilution is adding solvent to a solution to lower its concentration. Since water is the most common solvent, it often means adding water.
Let’s consider an example where we have 100.0 mL of a 5.0 M NaOH solution. If we were to dilute the sample by adding 400.0 mL of water, what would the final molarity of the NaOH solution be?
Initially there are a certain number of moles of NaOH in the solution. When we dilute with water, we are not adding any more NaOH. so that means that
moles NaOH before dilution = moles NaOH after dilution
since molarity = moles/volume:
,
we can also say that .
Therefore, since moles NaOH before dilution = moles NaOH after dilution,
this is usually written as the dilution equation
For our example, M1= 5.0 M, V1 = 100.0 mL, and V2=500.0 mL (V2 is the volume after dilution, where the 400 mL is added to the 100 mL).
Substituting into the equation, . Solving for M2 yields M2 = 1.0 M.
Notice that, even though volume is in liters for molarity, mL were used to solve this problem. As long as the same units are used for V1 and V2, this is acceptable.
Objective 11 practice
Objective 12: Calculate the mass of a substance produced or used in precipitation or neutralization reaction.
Stoichiometry
You can identify a stoichiometry problem when you are working with a known or given chemical reaction, are given information that will allow you to determine the number of moles of one of the reactants or products, and you wish to determine information about one of the other reactants or products. All stoichiometry problems involve the same three steps:
- Use given information to calculate moles of a reactant or product.
- Use the chemical equation and mole ratio to go from the moles of a reactant or product in step one to the moles of reactant or product you are asked about
- From the moles of reactant or product from step 2, complete the problem by calculating the answer.
You were introduced to one type of stoichiometry problem in the last unit (in Chapter 3). This was a mass-mass stoichiometry problem. For those problems, the three steps were:
- Given grams to moles of a reactant or product
- Moles of a reactant or product to moles of another reactant or product using the chemical equation and mole ratio
- Moles from step 2 to grams
In solution stoichiometry problems, steps 1 and/or 3 can be replaced by a molarity calculation. Figure 4.11 in OpenStax shows a roadmap or flowchart for these stoichiometry problems, as well as mass-mass and other variations.
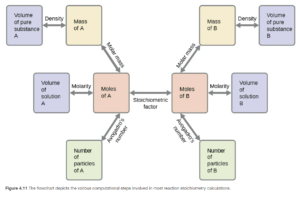
On the roadmap, A is the reactant or product you are given information about, and B is the reactant or product you are asked about. Let’s look at an example:
When solutions of lead (II) nitrate and sodium sulfate are mixed, lead (II) sulfate precipitates out of solution according to the equation
If 2.00 L of 0.0250 M Na2SO4 is mixed with a solution containing an excess of Pb(NO3)2 , calculate the mass of precipitate formed.
The three steps will be:
Step 1:Use given information to calculate moles of a reactant or product.
The given molarity and volume of Na2SO4 can be used to calculate moles of Na2SO4
Step 2: Use the chemical equation and mole ratio to go from the moles of a reactant or product in step one to the moles of reactant or product you are asked about
You are asked about PbSO4 precipitate. Therefore use the mole ratio of Na2SO4 to PbSO4 in the balanced equation, which is 1:1
Step 3: From the moles of reactant or product from step 2, complete the problem by calculating the answer.
Use the moles of PbSO4 and the molar mass of PbSO4 to calculate the mass of precipitate.
The three steps are shown in the following dimensional analysis:
Again, you are not required to use dimensional analysis. If you would prefer to do the three steps algebraically on by other means it is acceptable, as long as you can do it correctly and show your work.
Objective 12 Practice
When solutions of silver nitrate and calcium chloride are mixed, silver chloride precipitates out of solution according to the equation
27.0 mL of 6.0 M sulfuric acid is spilled on a lab bench. You can neutralize the acid by sprinkling NaHCO3 on it and mopping up the resultant solution. The reaction is given below:
Objective 14:Identify a chemical reaction as dissociation, combination, decomposition, combustion, neutralization, single replacement, double displacement. (See the Chemical Reaction Handout.)
Objective 15: Given the reactants and the reaction type (dissociation, combination, decomposition, combustion or neutralization, single replacement, double displacement), predict the products and then balance the equation. (See the Chemical Reaction Handout.)
Combination, decomposition, and combustion reactions were covered in Unit 1. You should still be able to recognize those reaction types and predict their products on quizzes and exams in Unit 2. Dissociation reactions were covered at the beginning of this topic in Objective 1.
Double replacement or double displacement reactions were covered earlier in Objectives 4 and 5 (I just didn’t tell you they were double replacement at that time). Precipitation reactions are examples of these reactions. Let’s take another look at our precipitation reaction example from unit 5:
In this (and other) double replacement reaction, the cations and anions “switch” Each cation from one reactant os paired with the anion from the other reactant to form the products. Lead (II) ion is with nitrate and potassium ion is with iodide in the reactants, but lead (II) ion is with iodide and potassium ion is with nitrate in the products. You should be able to write molecular, ionic, and net ionic equations for these.
This leaves neutralization and single replacement.
Neutralization Reactions
Acid base neutralization reactions are, like the precipitation reactions we have seen, double replacement reactions. An example is:
Typically we do not write the combination of the hydrogen ion from the acid and the hydroxide ion from the bases as “HOH”, though, we instead write it as:
These reactions have the general form:
acid + base →salt + water.
Like the precipitation reactions we have seen, you can also write molecular, ionic, and net ionic equations for these.
Go to the Chemical Reactions Handout on the course D2L site. To find it click “content”, then click “Reaction Handout CHEM 151”, then click “CHEM 151 Reaction Handout”. Neutralization reactions are discussed in detail along with precipitation equations in Section 6 of the Handout beginning on page 33 of the handout. Included are step by step examples on how to identify, predict and write these equations, a section on common errors, and practice questions with answers.
Single Replacement Reactions
Go to the Chemical Reactions Handout on the course D2L site. Single Replacement reactions are explained in detail in Section 4 of the handout beginning on page 17. Included are step by step examples on how to identify, predict and write these equations, a section on common errors, and practice questions with answers.
Objective 8: Define and identify end point, Stoichiometric point and indicator.
An acid-base titration uses acid base neutralization reactions in chemical analysis of materials. The link will take you to a section (called Titration) in OpenStax that gives you an excellent overview. Take a look at that link to get a feel for the equipment.
Titrations are described by saying that the analyte is titrated with the titrant. Therefore, in a titration of a strong acid with a strong base, the acid would be in the flask and the base would be in the burette.
In any titration, since an acid is reacting with a base (and at least one of them, if not both, is strong), an acid-base neutralization will occur:
acid + base —> salt + water
In a titration, the equivalence point (or stoichiometric point) is reached when the exact amount of titrant needed to neutralize the analyte has been added; also known as the stoichiometric point. This is driven by the coefficients in the balanced equation — is is often 1:1 (one mole acid to one mole base), but can be 1:2, 2:1, or others. Although the experimenter wants to knoe the equivalence point in a titration, it can’t be seen visually! We simply can’t see when the very last hydrogen ion is consumed by hydroxide (or vice versa). But what we can see is an endpoint. The endpoint is an observable signal that shows the equivalence point has been reached. There are many ways endpoints can be observed. Discussing them all is beyond the scope of this class. The most common one, however, is an indicator color change. An indicator is a substance added in a small amount that changes color at the equivalence point. You cannot see the equivalence point, but you can see the endpoint. In a well designed titration experiment, the endpoint will be as close as possible to the equivalence point.
Since the equivalence point is a stoichiometric point related to the balanced neutralization equation (telling us when the reaction has gone to completion) , titration analysis problems can be done just like any other stoichometry problem. Try the practice problem below:
Objective 8 practice
Sulfuric acid, H2SO4 reacts with NaOH according to the equation:
What’s next?
That concludes our discussion of solution chemistry for now. We will revisit other aspects of solutions later in the semester. We will also look at acid base chemistry in more detail later in the semester. But for now, we will move to our next topic, which is gases.