Objective 1: Define terms such as energy, work, kinetic energy, potential energy, system, surroundings, state function, and enthalpy.
Objective 2: State and apply the first law of thermodynamics.
Objective 3:Relate changes in enthalpy to changes in internal energy.
Objective 4: Distinguish between exothermic and endothermic processes.
These first 4 objectives are largely basics and definitions that we will use to actually do thermochemistry calculations and draw conclusions in the remaining objectives. You can read more about these basics and definitions in OpenStax Section 5.1
Energy, work, and heat
Energy (E) is the ability to do work or produce heat. Therefore, we can think of heat and work as two forms of energy.
Energy used to move an object over some distance is work (w). w = F × d, where w is work, F is the force, and d is the distance over which the force is exerted.
Heat (q) is the transfer of energy between 2 objects due to a temperature difference. Heat flows from the higher temperature object to the lower temperature object. Note heat is signified by the letter q (or Q), not H, as H is reserved for something else (we will see later).
Units of Energy:
The SI unit of energy is the joule (J). A Joule is not a large amount of energy. If we consider the energies associated with chemical reactions, there are usually thousands, tens of thousands, or hundreds of thousands of joules involved per mole of reactant. Therefore, we often use kJ in discussing energies associated with chemical reactions. In this topic, we will primarily use J and kJ for units of energy.
The calorie (cal) is another energy unit. It is used widely in chemistry, biology, and biochemistry. A calorie is defined as the energy needed to increase the temperature of 1 g of liquid water by 1 degree C.
The conversion between calories and joules is:
1 cal = 4.184 J
When you think of calories, you most likely think of food or nutrition.
In nutrition, the Calorie (C is capitalized) is actually a kilocalorie or 1000 calories.
1 Cal = 1000 cal = 1 kcal.
Kinetic and Potential Energy
Typically students first see the idea and concepts of kinetic and potential energy in physics. In physics, kinetic energy is the energy of motion. The kinetic energy of a moving object is defined by KE=1/2mv2. We encountered this in the previous topic when looking at kinetic molecular theory of gases. In chemistry, we can think of kinetic energy as the thermal (related to temperature) motion of molecules. It is proportional to the absolute (Kelvin) temperature.
In physics, we think of potential energy as stored energy. We sometimes think of it as energy of position, as an object that is elevated can have that stored energy converted to kinetic energy if it is dropped. In chemistry, potential energy is also the energy of composition. Different chemical bonding arrangements have different potential energies. In a chemical reaction, breaking bonds requires energy, forming bonds releases energy. So we can think of potential energy as being stored in bonding arrangements or chemical bonds.
System and Surroundings
The system is defined as the collection of matter we are evaluating. Since this is a chemistry class, the system will usually be a chemical reaction.The surroundings is everything in the universe that’s not in the system.
The internal energy of a system is the sum of all kinetic and potential energies of all the atoms and molecules of the system. We cannot calculate total internal energy. Instead we always look at the change in energy between our initial state and final state (ΔE; read as “delta E”).
How do we measure that change in energy? The energy leaving the system and going into the surroundings or coming into the system from the surroundings will do so as heat, work, or a combination of the two.
Energy flow between System and Surroundings
If energy flows from the system to surroundings, the final energy of the system is lower than the initial energy.
Mathematically, ΔE = Efinal-Einitial is negative in this case. The negative sign only tells us the direction of energy flow (system to surroundings). This energy flow could be as heat (system puts out heat to surroundings) , work (system does work on surroundings), or a combination of the two. Let’s look at an example
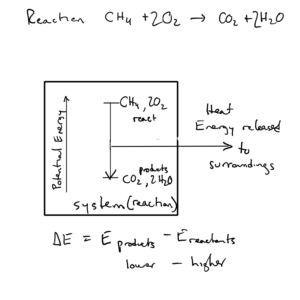
In this reaction, methane (the main component in natural gas) is burned.The reactants (1 mole methane and 2 mol oxygen) have higher potential energy due to their composition than the products (1 mol carbon dioxide and 2 moles water). The system goes from a state of higher to lower potential energy. That energy is lost to the surroundings as heat, or, as we know, the chemical reaction of burning natural gas produces heat and sends it out to the surroundings.
Similarly, if the products of a reaction are higher in potential energy than the reactants, energy (as heat or work) must go from the surroundings into the system for the reaction to occur. These reactions require energy. For these reactions, ΔE is positive, and the system absorbs heat from surroundings, and the surroundings does work on system.
The Law of Conservation of Energy (First Law of Thermodynamics)
The First Law of Thermodynamics states that energy cannot be created or destroyed (so it is also known as the the Law of Conservation of Energy). Even though energy is conserved, it can be converted from one type to another. As an example, kinetic energy can be converted to potential or vice versa. Or as we just saw, chemical potential energy can be converted to heat, which could raise temperature, increasing thermal kinetic energy of molecules.
Even though energy can flow from system to surroundings or surroundings to system, The total energy of the universe is a constant. If the system loses energy, it must be gained by the surroundings, and vice versa.
Mathematically the First Law can be stated as
where
- Δ = greek letter delta, signifies “change in”
- ΔE is change in internal energy
- q = heat
- w = work
Objective 2 Example:
Calculate ΔE for a system that releases 198 J of heat and the surroundings does 89 J of work on the system.
We can think of this in one of two ways. First, we can keep track of which way the energy is flowing. 198 J is leaving the system as heat. 89 J is being done by the surroundings on the system (entering the system as heat). Therefore with 899 J going in and 198 J going out, the net result is 109 J leaving the system.
Alternatively, we can use mathematical sign:
q = -198 J since heat is leaving the system.
w – +89 since the surroundings is doing work on the system
=-198+89=-109J
As long as you can identify the direction of energy flow, either by words (“leaving the system, producing energy”) or sign (negative), it is acceptable.
Objective 2 Practice:
State Functions
State functions are path independent (not path dependent). The value of a state function does not depend on how the substance got to that state. Examples of state functions are temperature, kinetic energy, potential energy, internal energy, pressure, and volume.
Functions that are path dependent are NOT state functions. Their value does depend on path. Heat and work are examples.
As we will see later, the concept of state functions will be useful in allowing us to calculate the heat required or released by a chemical reaction if we are provided with certain common data.
The idea of state functions is somewhat abstract; OpenStax Figure 5.20 and the text around it gives a nice illustrative example.
Enthalpy (H)
Enthalpy is a state function, so its value is independent of path. The enthalpy of a system is defined as:
H = E + PV
where H is enthalpy, E is internal energy, P is pressure, and V is volume.
Enthalpy is a function of convenience since:
q=ΔH at constant pressure.
OpenStax section 5.3 (read the section just below the mountain picture) shows mathematically why this is the case.
What is convenient about q=ΔH at constant pressure?
Heat flow is easy to measure (we will discuss how in a future objective). Since chemical reactions are often run at constant pressure, we can measure the heat and know the enthalpy change, which is quite useful since enthalpy is a state function (take my word for it — we will see in a bit). We can do this without having to measure the work, which is not measured as easily.
In a chemical reaction – the flow of heat into or out of the reaction system is the change in enthalpy of the system and is called the enthalpy change of reaction or the heat of reaction. Note H is enthalpy. It is not heat. ΔH is just equal to heat, so we say “heat of reaction”.
Objective 5: Given the change in enthalpy for a reaction, calculate the heat transferred for a given amount of reactant.
ΔH or enthalpy change for reaction – also known as “heat of reaction”
ΔH = H final – H initial
Since for a reaction the reactants are the initial state and the products are the final state,
ΔH =H products – H reactants
If ΔH is positive
- H products > H reactants
- Heat is absorbed by system
- reaction requires energy
We call these reactions where ΔH is positive endothermic reactions. You can think of “endo” sounding like “in” and “thermic” is from thermal, which is related to heat. So endothermic means “heat in”, or heat is going into the reaction system (from the surroundings).
If ΔH is negative
- H products < H reactants
- Heat is released by system
- reaction releases energy
We call these reactions where ΔH is positive exothermic reactions. You can think of “exo” as being from exterior, meaning “outside” or “out”and “thermic” is again from thermal, which is related to heat. So exothermic means “heat out”, or heat is going out of the reaction system (to the surroundings).
Objective 5 Example
Consider the following reaction:
Calculate the heat produced when 8.18 g of CO react with an excess of Ni to give Ni(CO)4 in a constant pressure system
To start, you need to understand the convention in which heats of reaction are written. The ΔH of -163 kJ means that 163 kJ of energy is produced as heat (exothermic) when one mol Ni reacts with four moles of CO to produce one mole of Ni(CO)4. Therefore,
163 kJ are produced for every one mol Ni reacted
163 kJ are produced for every 4 mol CO reacted
163 kJ are produced for every one mol Ni(CO)4 produced.
We could write this as conversion factors if we want to use dimensional analysis:
Since we know the kJ produced per mole of CO, we can start by converting mass to moles of CO:
If one mol CO reacted produces 41.75 kJ, then to calculate what 0.292 mol produces, we multiply:
0.292 X 41.75 = 11.9 kJ energy produced.
Note I could either say “11.9 kJ produced” or -11.9 kJ.
Alternatively, we could use dimensional analysis:
Objective 5 Practice
Consider the following reaction:
Objective 6 – Using the relationship: q = Sp Ht x m x ΔT, determine the values of one of the variables given the values of the others.
As substances absorb heat, their temperature increases. As heat is removed or lost from a substance, its temperature will decrease. Different materials require different amounts of heat to cause a different temperature change, for example, metals will gain temperature more readily than materials like concrete or clay.
The amount of energy required to raise the temperature of an object or collection of matter by 1 K (or 1°C) is its heat capacity (C). For example, the energy required to raise the temperature of the water in a filled, 20 oz bottle would be the heat capacity of the 20 oz bottle of water. The energy required to raise the temperature of Lake Superior would be the heat capacity of Lake Superior.
It takes a LOT more energy to increase Lake Superior’s water temperature by 1 degree than it does the water bottle, because there is so much more water in Lake Superior. The heat capacity of an object depends on the size or amount of the substance. For this reason, a heat capacity is valid only for a specific object. Those objects are often calorimeters, which are devices used to measure properties in thermochemistry.
The heat capacity of 20 oz. water is 2475 J/ºC. Therefore, if we wanted to calculate the energy required to increase the temperature of the water in the bottle from 20.00 to 25.ooºC (a 5.00 degree increase), we would do so as follows:
(to three sig figs)
Either dimensional analysis or knowing the definition of heat capacity (it is the energy required to increase the temperature by one degree, so it would take five times as much to increase it by five degrees, allows us to complete the calculation. However, many students prefer an equation so we can also put it in equation form:
where
- q=heat (in J or kJ)
- C is heat capacity in J/ºC, kJ/ºC, J/K, or kJ/K
- ΔT is temperature change in ºC or K.
Using the equation for this example:
It is useful to also have a quantity that would. allow us to do these calculations without having to use a different heat capacity for each object. The quantity most often used is the specific heat capacity, Cs (or simply specific heat).
The specific heat is the amount of energy required to raise the temperature of 1 g of a substance by 1 K (1°C). Since specific heat does not depend on the amount of a substance, the specific heat of a pure substance (element of compound) can be known. We could remove one gram of water from our 20 oz bottle and one gram of water from Lake Superior, and it would require the same amount of energy to increase their temperatures by the same amount, because they are both water. Specific heat depends only on the composition, so we can look it up.
A similar equation for specific heat is:
where
- q=heat (in J or kJ)
- C is heat capacity in J/g⋅ºC, kJ/g⋅ºC, J/g⋅K, or kJ/g⋅K
- m is mass in grams
- ΔT is temperature change in ºC or K.
You may also encounter molar heat capacity, Cm , which is the amount of energy required to raise the temperature of 1 mole of a substance by 1 K (1°C) .
One caution when dealing with heat capacity or specific heat problems in Kelvin temperature: A temperature change of 1 degree C is also a temperature change of 1 K (not 274 K). Here’s why:
20°C = 293 K and 21°C= 294 K. So a change from 20°C to 21°C is the same as a change from 293 K to 294 K (1 degree in each case). For this reason either /°C or /K are acceptable in heat capacity or specific heat units, without changing the values.
Objective 6 Example:
The specific heat capacity of silver is 0.24 J/ g.°C. Calculate the energy required to raise the temperature of 150.0 g of silver from 0 °C to 25 °C?
Objective 6 Practice:
Objective 7: Solve calorimetry problems using heat gain equals heat loss and q = Cs m ΔT or q = C ΔT.
Calorimetry:
The concepts of heat capacity and specific heat allow the amount of heat flowing between a system and surroundings (in either direction) to be measured by measuring a temperature change. the technique of calorimetry uses measured temperature changes and specific heat/heat capacity calculations to determine heat flow associated with a chemical reaction or physical processes. The governing property in calorimetry is heat gained by the system (or one object) equals heat gained by the surroundings (or other object). The heat flow to or from the surroundings can be measured if the heat capacity or the specific heat and mass of the surroundings is known by measuring the temperature change of the surroundings.
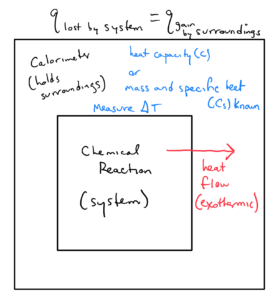
The above figure is a schematic for calorimetry. The reaction system is represented by the inner box. This example reaction is exothermic, so heat flow from the system to the surroundings is shown by an arrow leaving the system. As heat flows into the surroundings (the device containing the surrounds is known as a calorimeter), the temperature of the surroundings will increase. The mass and specific heat (or heat capacity) is known for the surroundings. The temperature increase in the surroundings can be measured. The heat flow into the surroundings can then be calculated by the specific heat or heat capacity equation. Since the heat lost by the reaction equals heat gained by the system, once we know the heat gained by the reaction we know the heat provided by the reaction.
Let’s work through an example:
Objective 7 Example:
A student mixes 50.0 mL of 1.0 M HCl and 50 mL of 1.0 M NaOH in a coffee cup calorimeter. The following reaction occurs:
The temperature of the resultant solution increases from 21.0 °C to 27.5 °C. Calculate the enthalpy change for the reaction in kJ/mol HCl, assuming that the calorimeter loses only a negligible amount of heat and that the total mass of the solution is 100.0g . The specific heat of the solution is 4.18 J/g °C .
In this problem, we will consider the reaction to be the system and the solvent water (which makes up the bulk of the total mass of the solution and its specific heat) as the surroundings. The temperature of this solvent wate is increasing, indicating the reaction is producing heat (exothermic).
Our overall relationship governing calorimmetry is
Q lost by reaction = Q gained by solution
In calorimetry, you will always have enough data for either the system or surroundings calculation — in this case we are given the magg, specific heat, and temperature change of the solution:
- mass = 100.0 g
- specific heat of the solution= 4.18 J/g °C .
- ΔT = 27.5-21.0 = 6.5 °C
Therefore:
The reaction produced 2700 J, which raised the temperature of the resulting solution.
The problem asked to calculate the heat of reaction in kJ/mol HCl. The number of mol HCl reacted was:
Therefore. the heat produced per mol HCl in the exothermic reaction is
We need to report the reaction as exothermic, either with word or mathematical sign, so a negative sign can be used:
Objective 7 Practice:
A 110. g sample of copper was heated to 82.4 °C and placed into 116.0 g of water at 22.3 °C. The temperature of water increased to 24.9 °C. What is the specific heat of copper? Assume heat gained by the calorimeter is zero (in other words, all of the heat lost by the copper goes into the water). The specific heat capacity of water = 4.18 J/g °C
HINTS:
- you can tell heat is flowing from the copper to the water by the fact that the water temperature increases.
- heat will flow from the copper to the water as long as the copper is at a higher temperature that the water. It will stop when the temperatures are equal. Therefore, the final temperatures of copper and water are the same.
- Start with Qlost by copper=Qgained by water.. You will be able to solve for one of these Q values, then you will know the other.
Objectives 8-10: Using the concept of state functions to find ΔHreaction
Earlier, you learned that state functions are path independent (not path dependent).Since H is a state function, its value does not depend on how the substance got to that state.
Since ΔH=Hproducts-Hreactants, and Hproducts and Hreactants are the same no matter how they came to be, any path that a reaction takes from reactant to product (whether one step or many, whether real or imaginary), will have the same enthalpt of reactants and products and the same ΔH. This is useful in calculating heats of reaction from other known values. We’ll look at 2 ways to calculate ΔHreaction using state functions:
- Hess’s Law (objective 8)
- Standard Enthalpy of formation (objectives 9 and 10)
Objective 8 – Calculate ΔH for a reaction given ΔH values for reactions that can be combined (Hess’ Law) to yield the reaction of interest.
Imagine, for some reason, you wanted to calculate the ΔH for the reaction A + 2B →C+E (I know you probably can’t imagine why right now, but bear with me!)
A + 2B →C+E (ΔH unknown)
But let’s say the ΔH for these reactions are known:
A + B →C+D (ΔH =10 kJ)
B+D →E (ΔH =5 kJ)
If both of these reactions occur in sequence, we can find what happens overall by adding them together:
A + B →C+D (ΔH =10 kJ)
B+D →E (ΔH =5 kJ)
______________
A+2B+D→C+D+E (ΔH =15 kJ)
Note since we are saying both reactions are occurring in sequence we add the ΔH values.
We can cancel the D from each side like a spectator ion to leave:
A+2B→C+E (ΔH =15 kJ).
We have determined the heat of reaction for A+2B→C+E. It doesn’t matter whether the two steps we considered actually occurred, since H is a state function.
Hints for Hess’s Law Problems:
Unfortunately, we can’t always find data for reactions that perfectly add up to give the reaction we want. But we do have two other tools at our disposal to help:
If you write the reaction backwards, the sign on ΔH changes
If you scale up the coefficients, scale up the ΔH
Given these manipuations, Hess’s Law can be quite powerful. Let’s look at an example.
Objective 8 Example:
Given:
use Hess’s law to calculate ΔH for the reaction
Objective 8 Practice:
Given:
Calculate ΔH in kJ for the following reaction
Objective 9: Calculate the change in enthalpy for a reaction given the standard enthalpy of formation of reactants and products.
Enthalpies of Formation:
The enthalpy change associated with the formation of a compound from its constituent elements is called the enthalpy of formation (ΔHf). The standard enthalpy of formation (ΔH°f) of a compound is the change in enthalpy for the reaction that forms one mole of the compound from its elements with all substances in their standard states (superscript o indicates standard state conditions).
What is meant by standard states?
In thermodynamics, standard states mean the following:
- T = 25 °C
- For gases, pressure = 1 atm
- For aqueous solutions (aq), concentration = 1 M
- liquids and solids are pure
- elements are in their form they typically exist at in standard conditions. For example:
- Iron: Fe(s) is standard. Fe(l) is not, since iron is a solid at standard state.
- oxygen: O2 (g) is standard state, as oxygen exists as a diatomic gas at standard state. O(g), O(l), O2(l) are not.
Standard heats of formation are tabulated in a variety of places. The values can vary slightly depending on the source, so if you get an answer that is close but slightly different that is likely the reason.
The problems on the practice exams and quizzes on D2L assume you use the table at this link. It is a two page link including a periodic table on the first page. The standard heat of formation values are on the second page.
Appendix G in OpenStax also has Standard Heats of Formation. You can fine them via Google search as well.
Using Standard Enthalpies of Formation: The standard enthalpy change for any reaction (ΔH°) can be calculated from the standard enthalpies of formation of the reactants and products using:
Why this works
Since H is a state function, any set of reactions that take us from reactants to products (whether real or not) will have the same (and correct) ΔH. Using standard heats of formation assumes the following path:
Reactants→elements in standard states→products.
Breaking the reactants into elements is the opposite of forming them, so the sign is switched (by subtracting the reactants). Forming the products from the elements is the definition of standard heat of formation, so it can be added (sign left the same). Therefore, we use products – reactants.
An example of the use of heat of formation follows:
Objective 9 Example:
Using standard enthalpies of formation, calculate the heat of reaction for:
From the table, the following heats of formation can be found:
| Compound | ΔHf° (kJ/mol) |
| CO2 (g) | -393.5 |
| H2O (l) | -285.83 (make sure to use the correct value as both liquid and solid are on the table) |
| C3H8 (g) | -103.85 |
Notice O2 (g) is not on the table. As an element in its standard state, its ΔHf° value is zero. It takes no energy to form O2 (g) from O2 (g). ΔHf° values are zero for all elements in their standard states.
This reaction is quite exothermic, which is to be expected for the combustion of propane!
Objective 9 Practice:
Using standard heats of formation, calculate ΔH° for the following reaction
Objective 10: Write equations that represent the standard enthalpy of formation of a substance.
As covered earlier, the standard enthalpy of formation (ΔH°f) of a compound is the change in enthalpy for the reaction that forms one mole of the compound from its elements with all substances in their standard states.