Don’t worry that we are starting with Objective 4 and covering them in a different order as that outlined in the syllabus – it works perfectly fine and makes more sense in my opinion – it allows us to focus on masses of elements and compounds first before going to chemical reactions
Objective 4: Given a chemical formula or name, convert among moles, grams, amu, molecules and atoms.
Objective 5:Given the name or formula of an element or compound, determine its molar mass.
Atoms, ions, and molecules are the individual particles that make up matter. They are very small — too small to work with on an individual level (although scientists are getting very close as nanotechnology advances). In pretty much all cases, though, both chemists and nonscientists in everyday life work with macroscopic amounts of matter we can hold and see — and measure in grams, kilograms, and larger. Therefore, when working with these macroscopic amounts, it is convenient to think of groups of particles.
The mole
The idea of grouping objects is not new to you — you are probably familiar with a grouping known as the dozen, which is a grouping of 12. When we think of a dozen, we think of eggs or donuts, but a dozen can be 12 of anything. A dozen elephants is 12 elephants. A dozen atoms is 12 atoms. But a dozen atoms is still very small, so we need a number bigger than 12 when dealing with atoms. The standard number used in chemistry to group things for amounts if substances is the mole.
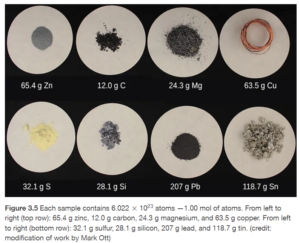
A mole is a grouping (just as a dozen is). Just a dozen equals 12 of anything, a mole is 6.022 X 1023 of anything. This number, 6.022 X 1023 , is called Avogadro’s number. You don’t really hear of it outside chemistry or physics because a mole of everyday objects large enough for us to see is almost unimaginably large. Why such a big number? Atoms and molecules are so small that we can only deal with large quantities of them. A dozen atoms of molecules is still an unimaginably small amount, but a mole of atoms or molecules is a reasonable amount we can work with. For example, a mole of water (6.022 X 1023 water molecules) is 18 mL (or about 1.2 tablespoons) of water.
Figure 3.5 in OpenStax (and the reading in the surrounding paragraphs and section on the mole) gives more detail and illustrations of a mole of various substances.
Definitions of the mole
- One mole = 6.022 X 1023 of anything
- Formal definition: A mole is the number equal to the number of carbon atoms in exactly 12 g of pure carbon –12
- Practical Definition: One mole of atoms of an element weighs the atom’s atomic mass in grams
Let’s look at the practical definition, which is how we will think of and use the mole for most of this class. We will start by looking at it in terms of elements and atoms before moving to compounds.
Atomic mass and moles of elements
1 carbon-12 atom has a mass of 12 amu. 1 mole of C-12 has a mass of 12 g. This one mole of carbon, with its mass of 12 g, is made up of 6.022X1023 carbon-12 atoms.
Therefore
1 mol C-12 =28.09 g C-12 = 6.022X1023 C-12 atoms
What if the element is not a pure isotope? For example, chlorine is made up of about 75% Cl-35 and about 25% Cl-37. A weighted average of the isotopes and their precise masses (you learned about this in the previous topic) gives an average atomic mass of of 35.45 amu. This is the mass found for Cl on a periodic table. This means that while no single chlorine atom has a mass if 35.45 amu, on average a Cl atom has a mass of 35.45 amu. If we have 6.022X1023 Cl atoms chosen at random, and we don’t purify them by isotope, the total mass of all those atoms (which is 1 mole of Cl will be 35.45 g. And the average mass of each individual atom will be 35.45 amu.
To summarize:
- 1 mol Cl =35.45 g Cl = 6.022X1023 Cl atoms
- The average Cl atom has a mass of 35.45 amu
Examples – Atoms, elements, and moles
In these examples, all atomic mass values from the periodic table will be rounded to 1 number after the decimal point. That will be acceptable for you do do in this class this semester.
Calculate the moles of sodium and the numbers of atoms of sodium in 10.0 g.
Atoms:
Note the following from the above example:
- you can also simply calculate the number of moles by simply dividing the grams by the atomic mass (10.0/23.0 = 0.435). You are not required to use dimensional analysis. I care that you can convert, not how (as long as you can show your work).
- Even though dimensional analysis is not required, if you are unsure whether you should multiply or divide (as beginning students often are), I would strongly recommend that you use it to help you. Do NOT try to memorize when to multiply or when to divide.
- The abbreviation “mol” is used for moles when showing units.. Never use “M” or “m” to describe moles. M and m will be used for other purposes later.
See Examples 3.3, 3.4, and 3.5 in OpenStax Section 3.1. It will show examples of the calculation of mass given moles (the above calculation in reverse) and more examples of conversions between mass, moles, and number of atoms.
Practice – Atoms, elements, and moles
Formula weight, molar mass and moles of compounds
The formula weight or formula mass can be calculated for any compound for adding up all of the average atomic masses of the atoms making up the formula. For example, CO2 has 1 C atom and 2 O atoms in its formula.
1 (mass of C) + 2 (mass of O) = 1 (12.0 amu) + 2(16.0 amu) = 44.0 amu.
Similarly, for Ca(NO3)2, which has 1 Ca, 3 N, and 6 O atoms in the formula:
1 (40.1) + 2 (14.0) + 6 (16.0) = 164.1 amu
CO2 is a molecular or covalent compound, since it is made up of all nonmetals. CO2 exists as molecules. Each CO2 molecule has a mass of 44.0 amu.
Ca(NO3)2 is an ionic compound. The calcium ion and nitrate ion are found in a 1:2 repeating ratio. While we say the formula mass is 164.1 amu, it is not the mass of a molecule as calcium nitrate doesn’t exist in molecules.
Whether a compound is molecular or ionic, the formula mass also gives us the grams in a mole. When it is in grams per mole, it is called the molar mass.
The molar mass of CO2 is 44.0 g/mol
The molar mass of Ca(NO3)2 is 164.1 g/mol
For CO2: 1 molecule has a mass of 44.0 amu. 1 mol CO2 has a mass of 44.0 g. There are 6.022 X 1023 CO2 molecules in 1 mole of CO2.
For Ca(NO3)2 : 1 mole Ca(NO3)2 has a mass of 164.1 g.
Conversions between moles, grams, and molecules are similar to those with atoms and elements.
Practice – Atoms, elements, and moles
Objective 6: Given the chemical formula or name of a substance, calculate its percent composition.
Percent composition (composition by mass)
Percent composition of a compound describes what percentage each element in a compound contributes to the compound’s total mass. It is best illustrated by an example. Let’s calculate the percent composition of tetraphosphorus decoxide, P4O10.
We determine the molar mass of P4O10 as follows:
4 (31.0) + 10(16.0) = 284 g/mol.
Of the 284 g, 4(31.0) or 124 g is from phosphorus and 10(16.0) or 160. g are from oxygen. Therefore,
A stepwise procedure outlining the steps above is:
- Compute the molar mass of the compound
- Calculate how much mass comes from each element (number of atoms of that element in the molecule x its atomic mass)
- Divide step 2 by step 1 and multiply by 100 to convert to percent
- Sum the individual mass percent values – they should total to 100% within round-off error.
You can read more about percent composition in OpenStax Section 3.2.
Percent Composition – practice
Objective 7: Determine the empirical formula of a compound from a molecular formula or percent composition.
Objective 8: Given the empirical formula and molar mass of a compound determine its molecular formula.
The empirical formula is the relative numbers and types of atoms in a molecule, written as a formula. Empirical formulas are written as the the lowest possible whole-number ratios. Molecular formulas give the actual numbers and types of atoms in a molecule. Let’s look at dinitrogen tetroxide (N2O4) as an example:
- N2O4 is the molecular formula. It exists as molecules containing 2 N atoms and 4 O atoms.
- NO2 is the empirical formula, as 1:2 is the smallest whole number ratio. 2:4 can be reduced to 1:2.
Sometimes (H2O is an example) the empirical and molecular formulas are the same.
Determining Empirical Formulas From Percent Composition
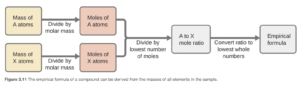
The empirical formula can be determined from the percentage composition of elements. It essentially involves converting the masses of all elements to moles, allowing for a conversion from a mass ratio to a mole ratio. The mole ratio gives the empirical formula. The procedure is as follows:
- Assume the compound has a mass of exactly 100 grams. You can then convert percent to grams
- Convert grams to moles of atoms for each element
- Divide each value of moles by the smallest of the values.
- (If needed) Multiply each number by an integer to obtain the smallest possible whole number ratio
Figure 3.11 in OpenStax shows this procedure as a flowchart:
Determining Molecular Formulas From Empirical Formula and Molar Mass
The percent composition or empirical formula alone is not enough to determine the molecular formula. For example, if the empirical formula is NO2, anything with a 1:2 ratio (N2O4, N3O6,N4O8, …) could be the molecular. One must also know the molar mass of the compound to know the molecular formula.
Objective 7 and 8 example
A compound is made up of 56.3% oxygen and 43.7% phosphorus by mass. Its molar mass is 284 g/mol. Determine the empirical formula and molecular formula.
Going through the four steps shown above, the empirical formula can be determined from the percent composition:
Step 1.Assume the compound has a mass of exactly 100 grams. You can then convert percent to grams
Start with 56.3 g oxygen and 43.7 g phosphorus
Step 2: Convert grams to moles of atoms for each element
oxygen:
phosphorus:
Step 3:Divide each value of moles by the smallest of the values.
oxygen:3.52/1.41 = 2.49
phosphorus:1.41/1.41 = 1
Step 4: (If needed) Multiply each number by an integer to obtain the smallest possible whole number ratio
The 2.49:1 ratio is very close to 2.5:1. If we multiply the 2.5:1 ratio we get a whole number ratio of 5 oxygen to 2 phosphorus, or P2O5. The empirical formula is P2O5.
We are given the information that the molar mass of the actual compound is 284 g/mol. The molar mass of the empirical formula is:
2 (31.0) + 5(16.0) = 142 g/mol
Dividing the molecular formula molar mass by the empirical formula molar mass gives a factor of 2:
We will need to multiply the empirical formula by this factor of 2 to get the molecular formula of P4O10 :
P2O5 X 2 will yield P4O10
Examples 3.11 through 3.13 in OpenStax section 3.2provide additional examples.
Objective 7 and 8 practice
Objective 1: Write a balanced chemical equation given a word description or the formulas of the reactants and products.
Chemical Reactions or Chemical Equations
Chemists use chemical equations to describe chemical changes. The reactants are written to the left of an arrow which points to the products on the right side of the arrow. Chemical formulas are used to represent the reactants and products.
A + B → C + D
where A and B are the reactants and C and D are the products.
Coefficients are the numbers written to the left side of chemical formulas of reactants or products in a chemical equation. The coefficients are the ratio in which reactants are consumed and products are produced. The coefficients can be thought of as individual molecules or as moles in this ratio, but they cannot be thought of as grams. Coefficients must be the smallest whole number ratio. If the coefficient is 1, it is not written.
In a chemical equation, we read the + sign as “reacts with” and the →as “produces”. States are sometimes shown as solid (s), liquid (l), gas (g) or aqueous solution (aq))
We would read the equation as
“In this reaction, 2 moles of hydrogen gas and 1 mole of oxygen gas react to form 2 moles of liquid water.”
Coefficients are NOT part of the chemical formula. The coefficient 2 in frony of the H2O means 2 moles of water are formed for every one mole of oxygen and 2 moles of hydrogen that react. There is no such compound as “2 H2O”.
Balancing Chemical Equations
To be balanced, a chemical equation must be both mass and charge balanced. First, the number of atoms of each element must be the same on the reactant side and the product side (mass balanced). Secondly, the overall total charge of the reactants must equal the overall charge of the products (charge balanced). To balance a chemical equation, only the coefficients in the reaction may be changed. By changing the coefficients only, it is ensured that the chemical identity of all the chemical species in the reaction is not changed by balancing the equation.
Again, coefficients should be the smallest whole number ratio. If you balance using fractional coefficients, you can multiply through by the denominator to make them whole numbers.
For further reading and examples on balancing equations, read the first half of OpenStax Section 4.1. Stop where the section “Equations for Ionic Reactions” begins.
Examples: Balancing Chemical Equations
Example 1
Unbalanced: C3H8 (g) + O2 (g) → CO2 (g) + H2O (g)
Balanced: C3H8 (g) + 5 O2 (g) → 3 CO2 (g) + 4 H2O (g)
Note the above equation is balanced since each side contains the same number of atoms for each element (3 carbon, 8 hydrogen, and 10 oxygen) and the same total charge (overall charge is neutral or zero on both the reactant and product side)
Example 2
Balance the following equation:
Example 3
Balance the following equation:
Objective 2: Identify a chemical reaction as combination, decomposition or combustion. (See the Chemical Reaction Handout.)
Objective 3: Given the reactants and the reaction type (combination, decomposition or combustion), predict the products and then balance the equation. (See the Chemical Reaction Handout)
An overview of these objectives is presented here. For more detail, see the file titled “CHEM 151 Chemical Reactions Handout Unit I” in the Unit 1 folder under content in D2L. There are practice questions with answers in the handout — practicing and trying those is the best way to learn to identify reaction types and predict products.
Combination Reactions
In combination reactions, two elements and /or compounds combine to form ONE compound.
Combination type 1: a metal element reacts with a nonmetal element to form an ionic compound
In this example, the metal sodium combines with the nonmetal chlorine (which is diatomic) to produce sodium chloride.
In this video, the product is predicted and the reaction written for the combination reaction of lithium metal and nitrogen gas
Combination type 2: a nonmetal oxide compound reacts with water to form an acid
Sulfur trioxide (SO3) is a nonmetal oxide because sulfur is a nonmetal. We can think of nonmetal oxides as “dehydrated acids” – just add water and you get an acid. It is a combination reaction as the two reactant substances combine to form one product.
Combination Type 3: a metal oxide compound reacts with water to form a base
Calcium oxide (CaO) is a metal oxide because calcium is a metal. We can think of metal oxides as “dehydrated bases” – just add water and you get a base. If you don’t know what a base is, we will study them later this semester. For now, there is another way to think of it:
metal oxide + water —> metal hydroxide
So in this example,
calcium oxide + water —> calcium hydroxide
Decomposition Reactions
Decomposition reactions are the reverse of combination reactions. In these reactions, a compound is decomposed into two or more substances (elements and/or compounds).
Decomposition Type 1: The decomposition of a molecular compound to form its elements
Why are the products not H2 and O? Keep in mind that there are seven elements which exit as diatomic gases. Hydrogen and oxygen are two of those seven. Write the correct formula for hydrogen and oxygen molecule products before and finally balancing the equation.
Decomposition Type 2: The decomposition of an ionic compound to form its elements
NOTE: The fluorine product is written as F2 because fluorine is diatomic, NOT because there are 2 fluorines in CaF2.
Decomposition Type 3: Decomposition of carbonates to yield oxides and carbon dioxide
A general form for decomposition of metal carbonates is:
Metal carbonate → metal oxide + carbon dioxide
Essentially, the carbonate reactant loses CO2 and the metal oxide is left behind.
Decomposition Type 4: Decomposition of metal chlorates to yield metal chloride and oxygen
The metal chlorate loses all its oxygen, leacving a metal chloride behind. Notice the oxygen is lost as O2 since oxygen is a diatomic gas.
Combustion Reactions
Definition and General Form
A combustion reaction involves the reaction of a substance with oxygen (usually from air). The most common combustion reactions involve compounds of the form CxHy (hydrocarbon) or CxHyOz reacting with oxygen to form carbon dioxide and water as the only products. The reaction produces heat. Burning of fuels such as natural gas, coal, propane, and gasoline are combustion reactions.
The general form of the complete combustion reaction for a hydrocarbon is:
CxHy + O2 (g) –> CO2 + H2O (l)
The general form of a combustion reaction for a compound made up of carbon, hydrogen, and oxygen is similar:
CxHyOz + O2 (g) –> CO2 (g) + H2O (l)
To write these reactions, the compound undergoing combustion and oxygen gas will be the reactants, and carbon dioxide and water will be the products. Balance the reaction after writing it out.
Objective 9: Calculate the mass of a particular substance produced or used in a chemical equation.
Stoichiometry deals with the quantities of substances consumed and produced in chemical reactions
Stoichiometry happens in the world we are familiar with…consider sandwiches with one and only one slices of bologna between two (no more, no less) slices of bread.
We are used to dealing with sandwiches and bread, so it is easy to determine how many slices of bread are needed to make 4 sandwiches:
We could treat it as a chemical reaction. The “sandwich reaction” is then:
where M is bologna (with an unwritten coefficient of 1 ) and B is bread (coefficient of 2 for the 2 slices). MB2 is the sandwich.
The reaction tells us there are 2 slices of bread for every 1 sandwich or
the 2:1 ratio is a stoichiometric factor, where the 2 and 1 come from the reaction coefficients. This would allow us to complete the above question using either a proportion (multiplying the number sandwiches by 2) or by dimensional analysis, where the stoichiometric factor is a conversion factor. The chemical reaction must be balanced for this to work.
We don’t find it necessary to use dimensional analysis or to show our work in any way when dealing with bread and sandwiches, as they are familiar to us and the numbers are usually simple (like 2,4,and 8). But it can be useful when dealing with a chemical reaction.
Any real chemical reaction, such as has the same constraints.
If I were to ask how many moles of oxygen gas were needed to make 1.236 moles of aluminum oxide (assuming you had enough aluminum), it is no different the sandwich question above — we just need to follow the stoichiometric factor from the recipe reaction:
The key is that the reaction coefficients apply to a mole ratio. They do not apply to a mass or gram ratio (for example, there is not a ratio of 4 grams Al to 3 grams O2, but there is a ratio of 4 moles Al to 3 moles O2)
Mass-mass stoichiometry
As mentioned above, the reaction does not give us a mass ratio. It gives us a mole ratio. But in the lab and in everyday life, we measure and deal with masses, not moles of substances. Mass-mass stoichometry problems must take into account both gram to mole calculations and mole ratio calculations. If we wish to know the mass of a reactant or product in a reaction that corresponds to a mass of another reactant or product, we need a three step process:
given mass → moles of the given substance →moles of the substance we are asked about → mass we are asked for.
For example, for the same reaction
,
how many grams of oxygen would react with 138 grams of aluminum to produce aluminum oxide? We are given the mass of Al and asked for the mass of O2. So the three step process will be:
given mass Al→moles Al→moles O2→mass O2
Objective 9 example
Answer the following question for the combustion of propane:
See OpenStax Section 4.3 for more on stoichiometry, including solved example problems and practice problems with answers.
Objective 10: Given the amounts of two or more reactants and the chemical equation, determine the limiting reagent and the theoretical yield of the product.
Normally, when we go to the kitchen to make sandwiches, we do not have the exact number of slices of bread to go with the number of slices of meat we have on hand. In other words, our reactants are not stoichiometrically equivalent. Let’s say we had 10 slices of bread and 8 slices of bologna. How many sandwiches could we make (given our previous sandwich rule)? The answer is 5.
The thought process to figure that out went something like this: The 10 slices of bread is enough to make 5 sandwiches and the 8 slices of bologna is enough for 8 sandwiches. Therefore I can only make 5 sandwiches.
Bread would be the limiting reactant because it would run out first. The bologna would be the excess reactant because I would have some left over. The limiting reactant always determine the amount of product produced.
Limiting Reactant (Limiting Reagent)
When chemical reactants are mixed, they usually are not stoichiometrically equivalent (the right amount of one reactant to go with another). In most cases you’ll run out of one reactant or the other (just as we ran out of bread in our sandwich example). The limiting reactant will run out first. There will be leftover excess reactant.
Stoichiometry using the limiting reactant determines how much product will be produced.
Limiting reactant practice
130.g C3H8 reacts with 400.g oxygen in the following reaction:
Objective 10 practice
130.g C3H8 reacts with 400.g oxygen in the following reaction:
- What is the limiting reactant?
- How much CO2 is produced in grams?
HINTS:
- First go through a three step calculation to determine how much CO2 can be produced by the 130.g C3H8
- Then go through a three step calculation to determine how much CO2 can be produced by the 400.g oxygen.
- Just as with the sandwiches, the one that produces the lesser amount of product is the limiting reactant (it is not necessarily the smaller amount of reactant).
- The lesser amount of product will be the amount produced.
Objective 11: Given the theoretical yield and the actual yield, calculate the percent yield.
The theoretical yield is the amount of product predicted from stoichiometry, taking into account limiting reagents. For the previous example, the theoretical yield is 330 g CO2. However, in the real world reactions typically do not go perfectly and the amount produced is less than the theorecical is produced. The actual yield is the amount of product actually obtained in the reaction.
The percent yield relates the actual yield (amount of material recovered in the laboratory) to the theoretical yield:
Objective 11 Example:
In the previous example, 330. g carbon dioxide is the theoretical yield. It is the amount of product formed when the limiting reactant is completely consumed. If 290. g carbon dioxide is actually produced, what is the percent yield of carbon dioxide?
% yield = 290. g/330. g X 100 = 87.9 %