Objective 1: Apply the Law of Conservation of Mass. (and 2 other laws)
In the previous chapter, you learned the difference between theory and law: A theory explains what happens, while a law describes or summarizes what happens. In other words, a law summarizes many scientific observations but makes no attempt to explain why. Early chemists conducted many experiments in which they reacted elements to make compounds, decomposed compounds into their elements, and others. They were careful to make sure they didn’t lose any matter when carrying out these experiments and to obtain precise mass data by careful weighing. Over time, three laws emerged as observations were made again and again:
Law 1: Law of Conservation of Mass
The Law of Conservation of Mass says that matter is neither created or destroyed. If a chemical change occurs with a collection of matter, the total mass of the matter after the change is the same as the total mass of the matter before the change. In other words, for a balanced chemical reaction, mass of reactants that react = mass of products that are produced. Let’s look at an example:
Methane burns by reacting with oxygen present in air to produce steam and carbon dioxide gas. We could write the reaction as
methane + oxygen → water + carbon dioxide.
Law 2: Law of Definite Proportions
This law is also known as the Law of Constant Composition. This law states that all samples of a given compound, regardless of their source or how they were prepared, are made up of the same proportions of elements.
Let’s look at an example. Pure water, no matter where it is from, consists of two elements: hydrogen and oxygen. And any amount of water consists of 8 mass parts oxygen for every 1 mass part of hydrogen.
9 grams of water is made up of 8 grams of oxygen and 1 gram of hydrogen. 18 grams of water is made up of 16 grams of oxygen and 2 grams of hydrogen. 900 lbs of water is made up of 800 lbs of oxygen and 100 lbs of hydrogen. The ratio is always 8:1
Similarly, carbon dioxide always has a mass ratio of 3 mass parts carbon to 8 mass parts oxygen.
A specific ratio exists for all chemical compounds.
Law 3: Law of Multiple Proportions
If 2 elements A & B can form more than one compound, then the masses of B that combine with one gram of A are in the ratio of small whole numbers.
Lets look at an example of the above law with two compounds consisting of hydrogen and oxygen: water and hydrogen peroxide. I’ll restate the law for that specific example:
If 2 elements hydrogen & oxygen can form both water and hydrogen peroxide, then the masses of oxygen that combine with one gram of hydrogen are in the ratio of small whole numbers.
| compound | mass of compound (g) | mass of hydrogen (g) | mass of oxygen (g) |
| water | 9 | 1 | 8 |
| hydrogen peroxide | 16 | 1 | 16 |
Notice the ratio of masses of oxygen that combine with 1 gram of hydrogen is 8:16 or a simple 1:2 ratio.
Another example of compounds that follow the law of multiple proportions are the various nitrogen oxides, including N2O, NO2, NO3, N2O5, and others.
Objective 2: State the basic postulates of Dalton’s Atomic Theory.
Recall a theory explains phenomena while a law summarizes observations (without explaining why). Dalton’s Atomic Theory explained the three laws we just looked at. It is stated as four postulates:
- Each element is composed of extremely small particles called atoms
- All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element is different from the atoms of all other elements.
- The atoms of one element cannot be changed into atoms of a different element by chemical reactions; Atoms are neither created nor destroyed in chemical reactions.
- Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms.
Dalton arrived at this theory to explain observations summarized by the 3 laws. Let’s look at a couple examples of how the theory explains the laws.
Law of Conservation of Mass – explained by postulate 3, and to some extent by postulate 1. Any time we have a chemical reaction or change, we can think of the atoms involved as a set of Lego bricks. We start with the reactants and take them apart and build something else (the products) even though the legos (atoms) are arranged differently their total mass is not changed is they can’t be created or destroyed.
Law of Definite Proportions – Postulate 4 (compounds have the same number and kind of atoms) and 2 (all atoms of a given element are identical.
Law of Multiple Proportions – Postulates 4 and 2 (like the Law of Definite Proportions) and Postulate 1. Because matter comes in atoms, and the smallest amount of an element you can have is 1 atom (not 0.5 atome or 0.001 atoms), only certain amounts of one element can combine with another (leading to the ratios).
Dalton’s Atomic Theory was cutting edge, early 1800s chemistry. Of course, scientists pressed on and work progressed to where modern atomic theory as gone much deeper, explaining what atoms are made of. Although it has been found that Dalton’s theory isn’t entirely valid (you will see that when studying nuclear reactions and chemistry) much of it is and it explains much of what we see in chemistry to this day.
You can read more about the three laws and Dalton’s theory, and see more examples, in OpenStax Section 2.1
Objective 3:Describe the studies made by Thomson, Millikan and Rutherford which helped develop our understanding of the subatomic nature of matter.
Science continued to progress throughout the 1800s as scientists investigated whether atoms were made of something even smaller. Three studies led to discoveries that carried chemistry much closer to where it is today.
Thomson’s Cathode Ray Tube (Discovery of electron)
The cathode ray tube was a glass tube containing metal electrodes from which most of the air has been evacuated. When connected to a high voltage power supply, a glowing area was seen emanating from the cathode (see Figure 2.6 OpenStax).
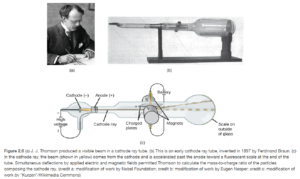
The cathode rays moved from the negative cathode to the positive anode, so Thomson knew they were made of something negatively charged. By deflecting them with varying magnetic and electric fields, he was able to determine their mass to charge ratio of the particles making up the rays. He also found the same results with every metal electrode used, indicating these negative particles were in all materials. These negative particles making up the rays were called electrons.
Millikan’s Oil Drop (determined charge and mass of electron)
Thompson’s experiment determined the charge/mass ratio of the electron. This experiment determined the separate values for the charge and the mass. Millikan’s experiment (see Figure 2.7 OpenStax) utilized charged, very small oil drops. The drops fell through the instrument due to gravity.
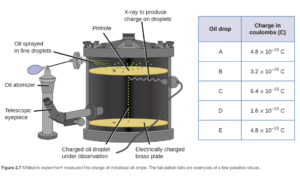
By adjusting the electric field, Millikan was able to slow the falling charged oil drops. Calculations based on the electric field and rate of falling determined the charge on the oil drops and eventually the electron. Now that the electron’s charge was determined, and the charge to mass ratio was know, the electron mass could be calculated as well:
Charge of electron: -1.6 X 10-19 C. (C is charge in Coulombs)
Mass of electron: 9.1 X 10-28 g
Atoms were known to be neutral in charge. Since negatively charged electrons were known to exist in atoms, there had to be a positive portion of the atom to cancel the charge from the electrons. But what the positive stuff was was unknown. Various models were proposed, including the plum pudding model, in which the atom consisted of a positively charger cloud or space with the negative electrons spaced throughout it (since we aren’t so familiar with plum pudding today, I prefer the “chocolate chip cookie dough” model) or Nagaoka’s model with a positively charged sphere surrounded by a ring of electrons.
The next breakthrough (Rutherford’s gold foil experiment), led to a better understanding of the positive part of the atom.
Rutherford’s Gold Foil (Discovery of Nucleus)
In the gold foil experiment (see Figure 2.9 OpenStax), Rutherford shot alpha particles at a thin sheet of gold foil and observed the pattern of scatter of the particles.
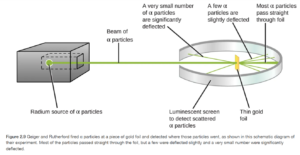
Almost all of the alpha particles went straight through, but a few alpha particles were deflected at an angle as they passed through or were even blocked by the gold foil. The alpha particles were known to be much smaller than gold atom but much smaller than electrons. The only explanation for the fact that most of the alpha particles traveled through the gold foil (which was about 2000 atoms thick) was that the gold atom was mostly empty space. The only explanation for the fact that some of the alpha particles were deflected was that occasionally they were repelled or deflected by some other positively charged particle in the atom when they got too close. This positive particle must be very small as the atom was mostly empty space. This small, particle (which contained all the positive charge and 99.99% of the atom’s mass) was called the nucleus. How small is the nucleus? If the atom was the size of Spartan Stadium, the nucleus would be about the size of a fly.
The positively charged particles making up the nucleus were called protons. Each proton had a positive charge of 1.6 X 10-19 C (equal in magnitude to the electron’s negative charge) and a mass equal to that of about 2000 electrons. The protons did not account for all of the mass of the nucleus, though, and that was not accounted for until the discovery of the neutron, which had a neutral charge but about the same mass as a proton.
Objective 4:Describe the composition of the atom in terms of the protons, neutrons, and electrons and the properties of these subatomic particles.
The atom is mostly empty space, with the tiny, positively charged nucleus at its center providing most (about 99.9%) of the mass. The nucleus is made up of protons, which are positively charges, and neutrons, which are neutral. Protons and neutrons have approximately the same mass. The electrons, move through the otherwise empty space around the atom. They are negatively charged and have a mass about 1/2000th of a proton or neutron.
Their masses and charges are as follows:
| Particle | mass (g) | charge (C) |
| proton | 1.67262 × 10−24 | +1.602 × 10−19 |
| neutron | 1.67493 × 10−24 | 0 |
| electron | 0.00091× 10−24 | -1.602 × 10−19 |
Notice that the magnitude of the charge is equal for the proton and the neutron. That means a neutral atom must have the same number of protons and electrons for the charges to cancel.
These very small masses and charges are inconvenient to work with, so scientists typically work with units for mass and charge that are more convenient.
1 atomic unit of charge = 1.602 × 10−19 C
1 atomic mass unit (amu) =1.6605 × 10−24 g
In atomic units, the masses and charges of the particles are:
| Particle | mass (amu) | charge |
| proton | 1.00727
(about 1) |
+1 |
| neutron | 1.00866
(about 1) |
0 |
| electron | 0.00055
(often neglected when estimating mass) |
-1 |
Objective 5:Write the nuclear symbol of an isotope or, given the nuclear symbol, state the mass number, atomic number, and number of protons and neutrons.
Nuclear Symbols
A nuclear symbol can be a shorthand way to represent a nucleus or an atom. An example of a nuclear symbol is:
In this symbol, the upper left number (23) is the mass number (symbolized by A). It is equal to the number of protons plus the number of neutrons.
The lower left number (11) is the atomic number (symbolized by Z). It is equal to the number of protons.
The element symbol (Na) is the symbol for the element sodium (atomic number 11). The element is identified by the atomic number. Since the 11 and Na are redundant, the symbol for sodium-23 can be represented in various ways:
Elements are listed by atomic number on the periodic table. For example, atomic number 6 is carbon. Therefore all carbon atoms have 6 protons and all atoms with 6 protons in their nucleus are carbon.
Remember, since the charges of a proton are equal but opposite, the number of protons and electrons must be equal in a neutral atom.
Try the following practice questions:
Isotopes
In the above practice, you looked at Cl-35 and Cl-37. They are examples of isotopes. Isotopes are atoms with same atomic number (17 in this esample) but different mass number (35 vs 37). They have the same number of protons (17 in this example) and different numbers of neutrons (18 vs 20). They are identified by their mass numbers (protons + neutrons).
Most elements have more than one isotope. A few examples are listed in OpenStax Table 2.4. You can read more about isotopes and examples in the surrounding paragraphs.
Ions
As seen earlier, sodium is element number 11, making 11 its atomic number. That means a sodium atom contains 11 protons. A neutral sodium atom must then have 11 electrons. An ion is a charged particle. Ions are formed when atoms gain or lose electrons.
| Particle | symbol | number of protons | number of electrons | Charge |
| sodium atom | Na | 11 | 11 | 0 |
| sodium ion | Na+ | 11 | 10 | +1 |
| oxygen atom | O | 8 | 8 | 0 |
| oxide ion | O2- | 8 | 10 | -2 |
From the table above, note the following:
- Some atoms form cations, which are positively charged ions. Sodium is an example. Elements on the left side of the periodic chart (metals) tend to form cations.
- Some atoms form anions, which are negatively charged ions. Oxygen is an example. Elements on the left side of the periodic chart (metals) tend to form cations.
- Ions can only be formed by gaining or losing electrons, not protons. If the number of protons change the element’s identity changes.
Different elements tend to form ions with different charges. Many of them are shown in the Formula and Nomenclature Handout you can download in the content area of D2L. They are also shown on OpenStax Figure 2.29 .
Objective 5 Practice
Objective 6: Given the atomic masses and the relative abundance of all the naturally occurring isotopes of an element, calculate the average atomic mass in amu.
Average Atomic Masses (Atomic Weight)
In nature, the elements occur as a mixture of isotopes. The percentage of an element that is a given isotope is the isotope’s natural abundance. Masses and abundances of isotopes are measured with a mass spectrometer. (Click the link for the mass spectrometer and read the section of OpenStax beginning above it on “Atomic Mass” to learn more about how abundance is determined). The listed atomic weights in the periodic table are the average mass of that atom’s isotopes weighted by their natural abundance.
What is meant by “the average mass of that atom’s isotopes weighted by their natural abundance”? Let’s look at carbon as an example. Carbon has three isotopes naturally occurring in the following percentage.
| Isotope | abundance | approximate mass (amu) |
| C-12 | 98.9% | 12 |
| C-13 | 1.1% | 13 |
| C-14 | about 10-10% | 14 |
If asked to calculate the average mass, it might be tempting to calculate the average as
But this would be incorrect. If you look at the abundance, almost all the carbon that exists is carbon-12. Little more than 1% is carbon-13 and less than 1/1000th is carbon-14. So on average, we would expect, on average, for a carbon atom to have a mass just slightly above 12. We can calculate it as follows using a weighted average, where each isotope’s mass is multiplied by its abundance before adding them together:
If you look at the Periodic Table, the masses listed below each symbol is the average atomic mass. Note the 12.011 amu for carbon.
See example 2.4 in OpenStax Section 2.3 for another example of calculating average atomic mass.
Objective 6 practice
Objective 7: Given a periodic table, identify elements according to the following: metals, nonmetals, transition metals, halogens, noble gases, alkali metals, alkaline earth metals, periods, families and groups.
A periodic table is available at this link. This same link is also in the “Useful Links” section in the right sidebar of this website.
The periodic table contains the arrangement of elements in order of increasing atomic number.
The vertical columns are called Groups or Families. Several numbering conventions for groups are used, including from 1 to 18, or from 1A to 8A and 1B to 8B). Elements with similar chemical and physical properties are in the same column. For example, Li, Na, K, and Rb tend to have a +1 charge when they form ions. Some of the families have historical names that are commonly used by chemists, and you should recognize these: the Alkali Metals, Alkaline Earth Metals, Halogens, and Noble Gases. OpenStax Figure 2.27 illustrates these.
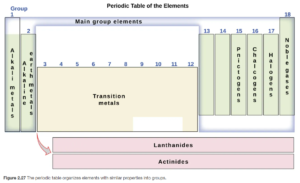
The horizontal rows are called Periods. Each period shows the pattern of properties repeated in the next period. The periods are numbered 1 starting with H, 2 starting with Li, 3 starting with Na, etc.
There is another useful online periodic table at this link. You might also find it useful to have a periodic table app on your phone for quick reference in this course – here’s a link to a nice app for Apple or Android phones.
Objective 7 Practice
Objective 8: Given the chemical formula of any atom, ion or compound listed in the “Formula and Nomenclature” handout, give the name and vice versa.
Your course D2L site contains a lot of resources to help in the study of chemical formulas and names, first and foremost the “Formula and Nomenclature Handout”. To access these:
- Go to the course D2L site
- Click Content
- Click “CHEM 151 Formulas and Nomenclature – Handout, Interactive Practice, and Drills”.
Use these resources to master your understanding of chemical formulas and names. Ultimately, understanding nomenclature is about much more than being able to write a formula given a name or vice versa. It will make the course much easier as it will help you understand and predict products of chemical change, masses of products, and many other aspects of chemistry, beginning with our next topic.
OpenStax Section 2.7 has additional information on nomenclature.
Objective 10: Given a molecular formula, indicate how many atoms of a given element are in the molecule.
Chemical Formulas
Chemical Formulas are the formulas you have been looking at in the Formula and Nomenclature Handout. All chemical compounds have chemical formulas, but the formulas may mean slightly different things depending on the type or classification of compound. These types and classifications (molecular vs ionic compounds) are discussed next. For further understanding, read OpenStax Section 2.6.
Molecular (covalent) compounds:
Molecular compounds typically consist of all nonmetal atoms. Examples include NH3, CO2, CH4, H2O2, C6H12O6, P2O5, and countless others. The smallest amount of a molecular compound you can have is a molecule. The chemical formula (H2O for example) indicates which atoms are found in the molecule (H and O), and how many of them (2 hydrogen and 1 oxygen) are in one molecule. One CO2 molecule would contain 1 carbon atom and 2 oxygen atoms. In molecular compounds, the atoms are bonded together by covalent bonding, in which the bonding atoms share electrons.
In ionic compounds:
Ionic compounds are made up of positive cations and negative anions existing in the necessary ratio to balance the charge. The simplest ionic compounds are made up of a metal cation and a nonmetal anion.
An example of an ionic compound is NaCl (sodium chloride). NaCl does not consist of individual molecules made up of one Na atom and one Cl atom. In NaCl, the sodium is Na+ ions and the chlorine is Cl– (chloride) ions. Rather than existing as molecules, they exist in a repeating structure called a crystal lattice. This lattice has a 1:1 ratio of sodium ions to chloride ions.
CaCl2 has a ratio of one Ca2+ ions to two Cl– ions. The 1:2 ratio is needed to balance the charges for a neutral compound.
More complex ionic compounds may contain a polyatomic ion. For examples and explanations, see the formula and nomenclature handout in D2L or OpenStax Section 2.6.
Structural Formulas
In structural formulas (sometimes used in molecular compounds), individual atoms are shown (no subscripts) and lines are used for chemical bonds between the atom that are bonded together. Using water as an example:
Chemical formula: H2O
Structural formula: H-O-H
Objective 9: Identify the elements that exist as diatomic molecules.
Seven elements in their pure form exist primarily as diatomic (2 atom) molecules. For example, the oxygen in air (or pure oxygen as well) is not made up of individual oxygen atoms but as O2 molecules. The seven diatomic elements are:
Hydrogen : H2
Nitrogen : N2
Oxygen : O2
Fluorine :F2
Chlorine :Cl2
Bromine :Br2
Iodine :I2
A mnemonic I use to help me remember them is:
Bruce Never Has Fun In Cleveland Ohio
What’s next?
In our next topic (stoichiometry), we will relate what we have learned about atoms, molecules, and formulas to masses of elements and compounds. Then we will apply that knowledge to chemical reactions.