Objective 4: Define the terms solubility, miscible and immiscible.
Solutions are homogeneous mixtures of two or more pure substances. They are made up of a solvent and one or more solutes. A solute is a substance dissolved in a solution and the solvent is the substance that dissolves the solute. Often, we can identify the solute as the substance that changes phase to enter the solvent’s phase – for example, solid salt dissolving into water to make saltwater. If the solvent and solute are in the same phase (a mixture of liquids or a mixture of gases) the substance that is present in a larger amount is considered the solvent. For example, since air (a solution) is 79% nitrogen, oxygen (20%) and all the other gases in air are considered solutes while nitrogen is the solvent.
Solubility is a measure of whether the solute will dissolve in the solvent and to what degree. Solubility can be described qualitatively– soluble means the solute will dissolve; insoluble means it will not. We looked at qualitative solubility of salts in this course earlier with solubility rules; we will look at qualitative solubility in this unit for other substances as well. Solubility can also be described quantitatively, where solubility is how much of the solute will dissolve in the solvent (in g/L or mol/L). This will be studied in CHEM 152.
The term miscibility is often used for liquid solutions — liquids are miscible if they will mix – in other words, one liquid dissolves into the other. They are immiscible if they will not mix. As an example, alcohol and water are miscible while oil and water are immiscible – we will see why shortly.
Objective 7: Solve problems involving mass percent, parts per million (ppm), parts per billion (ppb), mole fraction, molarity and molality.
Concentrations of solutions
To describe a solution, one needs to describe components and relative amounts. The terms dilute and concentrated can be used as qualitative descriptions of the amount of solute in solution. A dilute solution has a low concentration, while a concentrated solution has a high concentration.
Concentration is the amount of solute in a given amount of solution. Concentration can be described in many ways, including molarity — which you learned about earlier this semester.
Molarity
There are three variables in the above equation — as you did in Unit 2, given any two you should be able to solve for the third.
Other ways concentration can be described include the following:
Molality
Be careful with molality and note the denominator is solvent, not solution.
Mole Fraction
Mass percent
Parts per million (ppm)
Parts per billion (ppb)
For any of these, given all but one variable you should be able to solve for the remaining one. The following practice problems give you a chance to practice:
Objective 7 Practice
A solution is prepared by adding 5.84 g of formaldehyde, H2CO, to 100.0 g of water. The final volume of the solution was 104.0 mL. Answer the following questions:
And here are two more practice questions:
Objective 1: Explain the solution process (solvation).
Objective 2: Describe the energy changes that occur during solvation and relate these to particle interactions.
Solutions form when the strengths of the intermolecular forces between the solute and solvent are comparable to or greater than those that exist between solute particles themselves or solvent particles themselves. As the solution forms, the solvent pulls solute particles apart and solvates them.
Some examples:
- Alcohol dissolves well in water because even though alcohol has hydrogen bonding and water has hydrogen bonding, the alcohol and water also hydrogen bond with each other. Since the intermolecular force between alcohol and water is comparable to that of alcohol with itself and water with itself, alcohol will dissolve or mix well with water.
- Oil and water do not mix. Oils are primarily hydrocarbons, which due to the very small electronegativity difference between C and H are essentially nonpolar. Since the intermolecular force between oil and water cannot compete with the hydrogen bonding water molecules have with each other, solvation does not occur.
The process of dissolution can be broken into three steps (these are shown in OpenStax Figure 11.4):
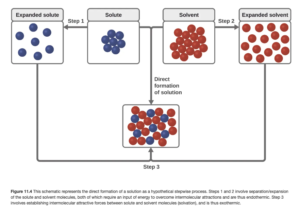
- separation of solute particles – endothermic
- separation of solvent particles – endothermic
- new interactions between solute and solvent and formation of solution – exothermic
If the intermolecular forces between solute and solvent are strong enough to compete with the intermolecular forces between each, step 3 will be sufficiently exothermic to make dissolution energetically favorable.
Even if it is not an exothermic process overall, slightly endothermic solvation often occurs. For example, NH4NO3 dissolves in water even though ΔHsolution = 26.4 kJ/mol (endothermic). This is because mixing or dissolving results in increasing disorder or randomness (known as entropy). Entropy will be discussed in CHEM 152, but a simple way to consider it is this: supposed I had two large buckets filled with marbles. One of the buckets had green marbles and the other white. I spilled them onto the floor, put on a blindfold, and picked up the marbles with the blindfold on and put them into the bucket. Do you think I would have them separated into green and white or would they be mixed up? You know what would happen! Similiarly, the tendency toward randomness means that the molecules can mix on the molecular level, even if it is slightly endothermic to do so.
Objective 3: Use the phrase “like dissolves like” to predict whether two substances will be soluble in each other.
The energetic and intermolecular force considerations just discussed in the previous objective can be summed up in a very simple guideline: “like dissolves like”.
The phrase “like dissolves like” can be used to predict solubility qualitatively. All substances can be put into one of two general groups:
- Ionic and polar
- Nonpolar
Substances in the same group (like) will mix or dissolve. Substances from different groups (unlike) will not mix or dissolve.
Objective 3 Practice:
Objective 5: Given the solubility of gas in a liquid at one pressure, determine the solubility at a different pressure using Henry’s Law.
Objective 6: Explain the effect of temperature and pressure on solubility.
Temperature Effects on Solubility
We will look at the temperature affects on solubility in aqueous solutions, though the following generally holds true for all solutions with liquid solvents. For solid or liquid solutes, the solubility may increase or may decrease as temperature increases, depending on the solute. However, the rate of solubility increases as temperature increases. In other words, however much will dissolve will dissolve faster in hot water – which is why we often think of heating to help with dissolution.
For solutions of gases in water, the solubility decreases as temperature increases. The reason for this is with the increased kinetic energy at the higher temperature, the gas more easily bubbles out of solution.
Pressure Effects on Solubility
The solubility of solids and liquids does NOT change appreciably with pressure. But pressure does affect the solubility of gases in liquids. Let’s look at that next:
Henry’s Law
Henry’s law applies to gaseous solutes in liquid solvents. It says that the amount of a gas dissolved in a solution is directly proportional to the partial pressure of the gas above the solution.
How would we put Henry’s Law into equation form?
As we saw earlier in our look at Charles’s Law in the topic of gases, the fact that solubility and pressure are directly proportional means (if pressure is doubled then solubility is doubled, if pressure is made three times smaller then solubility is three times smaller, etc ). This can be shown in equation form as:
where S1 and P1 are the initial temperature and volume and S2 and P2 are the final temperature and volume.
Objective 5 Example:
The solubility of O2 is 2.2 x 10-4 M at 0 °C and 0.10 atm. Calculate the solubility of O2 at 0 °C and 0.35 atm.
One approach is to think of this as a simple, direct, proportionality. The pressure is increased from 0.10 to 0.35 atm, getting 3.5 times greater. Therefore, the solubility will also get 3.5 times greater:
3.5 (2.2 x 10-4 M) = 7.7 x 10-4 M
Or, we can use the equation:
Rearranging to solve for the final solubility S2:
Objective 5 Practice:
The solubility of carbon dioxide in water is 0.161 g/100 mL and a partial pressure of carbon dioxide of 1.00 atm. A soft drink is carbonated with carbon dioxide at 6.00 atmospheres. What is the solubility (in g/100 mL) in water at this pressure?
Hint: use (g/100 mL) as the unit for concentration; do not multiply or divide the 100 mL in your calculation)
Objective 8:Describe the colligative effects of solute particles on the vapor pressure, boiling point, freezing point and osmotic pressure of a solution.
Colligative Properties
Colligative properties are properties of solutions that change when a nonvolatile solute is added to a solvent. The addition of the solute changes the properties of the solvent. These include:
- vapor pressure lowering ( Raoult’s Law) — addition of a nonvolatile solute lowers the vapor pressure of a liquid
- boiling point elevation — addition of a nonvolatile solute increases the boiling point of a liquid
- freezing point depression — addition of a nonvolatile solute decreases the freezing point of a liquid
- osmotic pressure — solutions with nonvolatile solutes have a property known as osmotic pressure
Colligative properties depend only on the number, not the identity, of solute particles in the solution. For example, when salt is added to water the freezing point is lowered (one reason the roads are salted in the winter). However, if one were to add the same number of particles (molecules) of sugar as particles (ions) of salt, the freezing point of water would be reduced by the same amount.
Next, we can look at (and see the equations for) each of these colligative properties:
Vapor Pressure Lowering
Consider two identical containers at the same temperature- one containing pure water and one containing a solution of sugar dissolved in water – for the sake of discussion lets say that 95% of the molecules are water and 5% are sugar. Both containers will have vapor pressure – as the water can escape into the vapor phase and the water vapor exerts pressure (you learned this in the previous chapter. But if we look at the sugar water solution, only 95% of the molecules can escape to form vapor – the sugar molecules will not escape to form sugar vapor! Sugar is a nonvolatile solute. The vapor pressure of the sugar will be 95% of the vapor pressure of the pure water since 95% of the particles (or molecules or moles) are water. This is described by Raoult’s law:
where
- PSolution is the vapor pressure of solution
- χsolvent is the mole fraction of the solvent (you learned to calculate mole fraction earlier)
- Psolvent is the vapor pressure of pure solvent
Figure 11.8 in OpenStax shows a schematic similar to the situation just discussed.
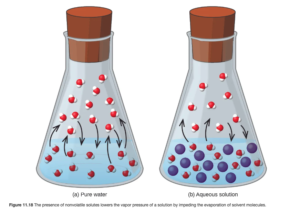
Boiling Point Elevation
We just saw that addition of a nonvolatile solute lowers the vapor pressure of a solution (Raoult’s Law). Therefore it will also increase the boiling point. Why? – the boiling point is the temp where Pvapor=Patmos. If Pvap is lowered, the temp must be increased for Pvapor=Patmos. Remember, as vapor pressure is decreased, boiling point is increased.
Boiling point elevation can be calculated by the following equation
- ΔTb = BPsolution – BPsolvent (it is the number of degrees the BP temperature is elevated or increased)
- Kb = BP elevation constant (°C•kg/mol or °C/m) – depends on the solvent – available in OpenStax Table 11.2)
- msolute = molality of the solute
Freezing Point Depression
In addition to increasing the boiling point, the addition of a nonvolatile solute lowers the freezing point. Therefore the nonvolatile increases the liquid range ( BP↑ , FP↓). The calculation is the same as for BP elevation. The only difference is the value of the constant Kf for freezing point is different than Kb for boiling point)
where:
- ΔTf = FPsolvent-FP solution (it is the number of degrees the BP temperature is depressed or decreased)
- Kf = FP depression constant (ºC•kg/mol or ºC/m) – depends on the solvent (available in OpenStax Table 11.2)
- msolute = molality of the solute
Osmotic Pressure
The osmotic pressure, π, can be calculated by
Where
- π = osmotic pressure
- M = molarity of the solution
- R = 0.0821 (atm∙L)/(mol∙K)
- T= temperature in Kelvin
I will discuss osmotic pressure more with a later objective.
Objective 9: Use Raoult’s Law to calculate a solution’s vapor pressure.
Objective 9 Example:
The vapor pressure of water at 25 °C is 23.76 torr. Calculate the vapor pressure of a solution of 43.0 g sucrose ( 342.3 g/mol) in 175 g water.
Since we are calculating the vapor pressure of a solution we will use Raoult’s Law:
where the solvent is water.
First, we can calculate the mole fraction of the solvent water. The most common error in vapor pressure lowering problems is to use the mole fraction of the solute.
Then we can calculate the vapor pressure of the solution:
The addition of the sucrose lowered the vapor pressure slightly to 23.76 torr.
See Example 11.6 in OpenStax Section 11.4 for another example of a vapor pressure lowering calculation.
Objective 9 Example: calculating vapor pressure with an ionic solution
The vapor pressure of water at 25 °C is 23.76 torr. Calculate the vapor pressure of a solution of 35.0 g Na2SO4 ( 142.0 g/mol) in 175 g water.
Since vapor pressure lowering is a colligative property, it depends on the number, not the identity, of dissolved particles. Since 1 mole of the soluble Na2SO4 provides 3 moles of ions:
Na2SO4 →2 Na+ + SO42-
that must be taken into account when determining mole fraction:
from, there we can calculate vapor pressure of the solution:
Objective 10: Calculate the boiling point and freezing point of solutions from colligative properties data.
Objective 10 Practice:
Ethylene glycol, the primary ingredient in antifreeze, has the chemical formula C2H6O2 and a molar mass of 62.1g/mol. Complete the calculations below for a solution 25.0 g of ethylene glycol in 100.0 g of water. Use the OpenStax Table 11.2 to get the Kb and Kf of water.
See Examples 11.7-11.9 in OpenStax Section 11.4 for another examples of these calculations.
Objective 12: Define osmotic pressure and calculate osmotic pressure, molarity or the van’t Hoff factor given information about the solution.
Osmosis and Osmotic Pressure
Osmosis is the flow of solvent through a semi-permeable membrane from solution. In osmosis, there is net movement of solvent from the area of higher solvent concentration (lower solute concentration) to the area of lower solvent concentration (higher solute concentration). Semipermeable membranes allow some smaller particles such as water to pass through, but blocks other larger molecules or ions which make up solute.
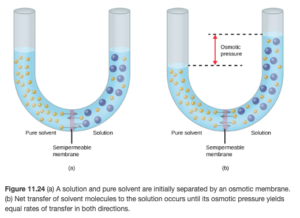
Osmotic Pressure (π=MRT) is the pressure that just stops the osmosis. The Greek letter pi is used to symbolize osmotic pressure – it is NOT the mathematical pi 3.14.
You may have heard of reverse osmosis, especially related to water filtration. In reverse osmosis, a pressure greater than the osmotic pressure is applied to force osmosis in reverse, producing pure (solvent) water on the left side of the diagram above.
Colligative Properties of Electrolytes
When ionic compounds dissolve in water, they dissociate – so the number of solute particles is a multiple of the number of moles of formula units. Colligative properties depend on the number, not the identity, of solute particles. The van’t Hoff factor, i, is the ratio of moles of solute particles to moles of formula units dissolved.
We saw earlier, when determining the vapor pressure lowering of Na2SO4, that 1 mole of Na2SO4 produces 3 moles of ions. The van’t Hoff factor i would be three.
The expected value of i can be calculated for a salt by noting the number of ions per formula unit:
i for NaCl = 2; i for K2SO4 = 3; i for Al(NO3)3 = 4.
Molecular compounds such as sugars and other compounds with formulas CxHyOz do not dissociate when they dissolve, so they have an i of 1.
Modified colligative property equations which include i
For electrolyte solutions, we modify the previous equations by multiplying by the van’t Hoff factor, i. Using these equations can help you remember to take the number of ions into account when solving colligative property equations with ionic solutes
Boiling Point elevation:
Freezing Point Depression:
Osmotic Pressure:
There is no modified form for vapor pressure lowering – it must be handled like the Na2SO4 problem on objective 9. Why? Because vapor pressure lowering uses the mole fraction of the solvent.
Objective 12 Practice:
Objective 11: Calculate molar masses and molalities from colligative properties data.
Any of the 4 colligative properties (vapor pressure lowering, boiling point elevation, freezing point depression, or osmotic pressure) can be used to determine the molar mass of a substance
In any problem where colligative properties are used to determine molar mass, the following four steps are followed:
- Step 1: use the appropriate equation from the colligative property
- Step 2: using the appropriate equation from step 1, solve for the concentration term from the equation.
- Step 3: solve for the number of moles of solute using the concentration
- Step 4: using the given number of grams of solute and the number moles from Step 3, solve for molar mass of solute
This process is similar for any of the colligative properties…….lets look at an example using boiling point elevation:
Objective 11 Example:
A 2.15 g of sample of molecular solid was dissolved in 25.0 g of CS2. The boiling point elevation of the solution was 1.59°C. Find the molar mass of the molecular solid. (Kb of CS2 is 1.07 °C /m).
Before starting the problem, make sure you correctly identify the solute and solvent. Since 2.15 g of sample of molecular solid was dissolved in 25.0 g of CS2., the solute is the molecular solid and the solvent is CS2.
Step 1: use the appropriate equation from the colligative property
The colligative property being used is boiling point elevation. The boiling point elevation equation is:
Step 2: using the appropriate equation from step 1, solve for the concentration term from the equation.
In the boiling point elevation equation , m (molality) is the concentration. Solving for molality:
Step 3: solve for the number of moles of solute using the concentration
The concentration determined in Step 2 is the molality. The molality equation is:
Therefore,
Remember, for this problem the solvent is CS2. Solving for moles of solute:
Step 4: using the given number of grams of solute and the number moles from Step 3, solve for molar mass of solute
Examples 11.11 and 11.12 in OpenStax Section 11.4 provide additional examples
Objective 11 Practice:
This concludes our discussion of properties of solutions. Please contact me should you have questions!