Before digging into the objectives for the topic of chemical kinetics in earnest, these first 2 objectives are catch-alls that will apply to the entire topic of kinetics.
Objective 1: Manipulate any mathematical equation involving logarithms.
Objective 2: Define or explain and apply the definition for the following terms: reaction rate, average and instantaneous rates, rate constant, reaction order, rate law, half-life, activated complex, activation energy, mechanism, elementary reactions (elementary steps), molecularity, rate determining step, catalyst (homogeneous and heterogeneous)
These objectives will be addressed throughout your study of kinetics. In acid-base chemistry, base 10 logarithms (log) are used heavily with p-functions such as pH and pOH. In kinetics, we will encounter many equations with natural logarithms (ln), which have a base e. We will work on solving the equations we need to study kinetics. If you need further help with these calculations, please do not hesitate to ask your instructor or tutors in the Learning Commons or elsewhere.
You will learn and use the terminology in Objective 2 throughout your study of kinetics. When you review this material, I would recommend that you check the list of terms in Objective 2 to make sure you understand these terms.
Objective 3: Distinguish among the average, instantaneous and initial rates for a chemical reaction.
Chemical Kinetics is the study of the rate (speed) at which a chemical process or reaction occurs. It deals with how fast reactants are consumed and products are produced in chemical reactions. The reaction rate is defined as the change in concentration of a reactant or product per unit time.
Units of rate
As just mentioned, reaction rate is defined as the change in concentration of a reactant or product per unit time. Therefore, its units will be in the form of:
These can take many different forms, including (but not limited to)
where M is molarity and s is time in seconds
, which is just another way to state M/s
…and many others.
We will most often use M or mol/L for the concentration portion. Seconds or minutes are usually the most convenient units of time when evaluating reaction rates, though days or weeks could be used as well.
Average rates
The reaction rate can be expressed as rate of disappearance of a reactant or rate of appearance of a product. Consider the reaction A→B. The average rate can be calculated as follows:
Why the negative sign for the rate of disappearance of A? Let’s look at an example: if [A] dropped from 5M to 3 M in 100 seconds, we could calculate as follows:
However, we would not say that A disappeared at a rate of -0.02 M/s. (similarly, we wouldn’t say someone lost -10 lbs on a diet!) the negative sign implies that the concentration of A decreased, or A is disappearing. The negative sign is there to make the negative delta A into a positive number when speaking of it as a disappearance. In other words, say
or that the rate of disappearance is 0.02 M/s, but don’t say both.
Average rate example
See Figure 12.2 in OpenStax, and read the paragraphs below it (down to and including Figure 12.3).
![alt="A table with five columns is shown. The first column is labeled, “Time, h.” Beneath it the numbers 0.00, 6.00, 12.00, 18.00, and 24.00 are listed. The second column is labeled, “[ H subscript 2 O subscript 2 ], mol / L.” Below, the numbers 1.000, 0.500, 0.250, 0.125, and 0.0625 are double spaced. To the right, a third column is labeled, “capital delta [ H subscript 2 O subscript 2 ], mol / L.” Below, the numbers negative 0.500, negative 0.250, negative 0.125, and negative 0.062 are listed such that they are double spaced and offset, beginning one line below the first number listed in the column labeled, “[ H subscript 2 O subscript 2 ], mol / L.” The first two numbers in the second column have line segments extending from their right side to the left side of the first number in the third row. The second and third numbers in the second column have line segments extending from their right side to the left side of the second number in the third row. The third and fourth numbers in the second column have line segments extending from their right side to the left side of the third number in the third row. The fourth and fifth numbers in the second column have line segments extending from their right side to the left side of the fourth number in the third row. The fourth column in labeled, “capital delta t, h.” Below the title, the value 6.00 is listed four times, each single-spaced. The fifth and final column is labeled “Rate of Decomposition, mol / L superscript negative 1 / h superscript negative 1.” Below, the following values are listed single-spaced: negative 0.0833, negative 0.0417, negative 0.0208, and negative 0.010."](http://fe2.openlcc.net/chem152/files/2024/01/Figure-12.2-300x114.png)
The data in Table 12.2 is for the reaction
Notice in Figure 12.2 that, as expected, the [H2O2] decreases as the reaction progresses. You should be able to calculate it over any of the time intervals using where A is H2O2.
Notice that the average rate changes depending on the time interval chosen, and that the rate of disappearance decreases over time. As the reaction goes forward, there are fewer collisions between reactant molecules, so the rate slows. This trend is true of most reactions.
Average rate practice
Consider the reaction .
The following data is tabulated as the reaction is carried out:
| Time (s) | [C4H9Cl] (M) |
| 0.0 | 0.1000 |
| 50.0 | 0.0905 |
| 100.0 | 0.0820 |
| 150.0 | 0.0741 |
Answer the following questions:
Instantaneous rate and initial rate
The average reaction rate (which are the ones you just calculated) is the change in the concentration of reactants or products over a period of time. The instantaneous reaction rate is the rate at a particular moment in the reaction. For example, if to had been asked to calculate the rate of disappearance of reactant at the instant of 100 seconds after the reaction started, that would be an instantaneous rate.
If one graphed the [C4H9Cl] on the y axis and time on the x axis, the instantaneous rate could be determined graphically from the slope at one point of the resulting curve (at whatever time you wanted to know the instantaneous rate). This is shown in OpenStax Figure 12.3. For those of you that have studied calculus, you might recognize it as the derivative at that point.
![alt="A graph is shown with the label, “Time ( h ),” appearing on the x-axis and “[ H subscript 2 O subscript 2 ] ( mol per L)” on the y-axis. The x-axis markings begin at 0.00 and end at 24.00. The markings are labeled at intervals of 6.00. The y-axis begins at 0.000 and includes markings every 0.200, up to 1.000. A decreasing, concave up, non-linear curve is shown, which begins at 1.000 on the y-axis and nearly reaches a value of 0 at the far right of the graph around 24.00 on the x-axis. A red tangent line segment is drawn on the graph at the point where the graph intersects the y-axis at 1.000. The slope is labeled as “slope equals negative capital delta [H subscript 2 O subscript 2 ] over capital delta t subscript 0 equals initial rate”. A vertical dashed line segment extends from the left endpoint of the line segment downward to intersect with a similar horizontal line segment drawn from the right endpoint of the line segment, forming a right triangle beneath the curve. The vertical leg of the triangle is labeled “capital delta [ H subscript 2 O subscript 2 ]” and the horizontal leg is labeled, “capital delta t.” The slope is labeled as “slope equals negative capital delta [H subscript 2 O subscript 2 ] over capital delta t subscript 12 equals instantaneous rate at 12 h.” A second red tangent line segment is drawn near the middle of the curve at 12.00 on the x-axis. A vertical dashed line segment extends from the left endpoint of the line segment downward to intersect with a similar horizontal line segment drawn from the right endpoint of the line segment, forming a right triangle beneath the curve. The vertical leg of the triangle is labeled “capital delta [ H subscript 2 O subscript 2 ]” and the horizontal leg is labeled, “capital delta t.”"](http://fe2.openlcc.net/chem152/files/2024/01/Figure-12.3-300x266.png)
The initial rate is the instantaneous rate near the beginning of the reaction (at t = 0).
Objective 5: Express the rate of a reaction in terms of the change in concentration of all reactants and products given the balanced chemical equation for the reaction.
Objective 6: Calculate the rate of formation or consumption for a reactant or product in a chemical reaction given the rate of formation or consumption any other reactant or product in the reaction.
If you have a balanced chemical equation, as long as you know the rate of disappearance or formation of any reactant or product in the reaction, you can then determine the rate of disappearance or formation of any other reactant or product in the reaction over the same time interval.
For example, for the reaction A→B + 2C, C will be produced twice as fast as B (for every 1 mole of B produced, 2 moles of C will be produced.
Therefore, if , then
.
We could also say
or . With this last equation, notice a coefficient of 2 for C results in multiplying
by 1/2.
Also notice that A is consumed at the same rate that B is produced (due to the 1:1 ratio). If then
.
We can express that as:
Let’s look at it with an actual example:
Consider the reaction:
The rate of disappearance of NO2 is same as the rate of appearance of NO and twice the rate of appearance of O2.We can write the relationship mathematically as
Note, the reactant NO2 has a negative sign to express it as a rate of loss. The coefficients of 2 from the reaction for NO2 and NO lead to the 1/2 in this equality for both. A coefficient of 3 would result in a 1/3. These equalities and their use are demonstrated in this video.
They are also demonstrated and this concept is discussed with another example in Section 12.1 of OpenStax, in the subsection titled “Relative Rates of Reaction”
Another Objective 5 and 6 example
Consider the reaction
In this reaction, the ratio of to is 1:1. The rate of disappearance of is the same as the rate of appearance of
.
Objective 5 and 6 practice
For the reaction , if
, determine the value of
.
For the reaction , if the concentration of hydrogen fell from 0.500 to 0.400 mol/L in 50.0 seconds, answer the following questions:
Objective 7: Determine the order of a reaction, rate law and calculate the rate constant k (and the units of k) for a reaction from a series of experiments given the measured initial rates for various concentrations of reactants.
Rate Law
The rate law for a reaction is an equation that relates reaction rate to a reactant concentration or concentrations. The rates of most reactions slow as the reaction progresses (see Figure 12.3 OpenStax) The reason for this is that most reactions have lower rates at lower reaction concentrations. The rate law enables the calculation of rate if the reactant concentrations are known. A rate law cannot be determined by looking at the chemical equation. It must be determined by experiment (or given in class).
Form of the rate law
For the reaction aA + bB →cC + dD, the rate law would have the form:
Where:
- n is order of reaction with respect to reactant A (or the “order in A”). Note n is not necessarily the same as coefficient “a”
- m is order in B. Note m is not necessarily the same as “b”
- n + m is the overall order of the reaction
- k is called the rate constant
Rate and rate constant are not the same thing. Again, as discussed in the earlier objectives, the rate of a reaction is the change in concentration with respect to time of a reactant or product. A rate constant (k) is a proportionality constant in the rate law. The rate constant does not depend on the concentrations of reactants but the rate does (if it is not zero order, which is something we will discuss later). If a question asks for rate constant, be sure you know it means k.
You can read more on this topic in the first half of OpenStax section 12.3.
Objective 7 practice
If the rate law for the reaction: is
, answer the following questions:
Using Initial Rates to Determine Rate Laws
For the reaction the rate law would have the form
The orders n and m cannot be determined simply by looking at the chemical reaction. Students are often tempted to get the orders from the reaction coefficients (because they are used to doing that for exponents in equilibrium constants), but this is incorrect.
How can the orders be determined, then? They must be determined from experimental data. The method of initial rates is an experimental method that does this.
In the method of initial rates, the initial rate for different starting reactant concentrations are measured. Only one reactant concentration is varied at a time so the effect of that reactant concentration (shown by the order in that reactant) can be determined.keep initial concentration of all reactants constant except one. If there are multiple reactants, this process can be repeated for each reactant. Once done, comparisons of the reaction rates resulting from the various reactant concentrations can be used to determined the orders and the rate law. Let’s see how in an example:
Objective 7 example
For the reaction 2 NO (g) + O2 (g), → 2 NO2 (g) the following data was collected:
| Experiment | [NO] mol/L | [O2] mol/L | Δ[NO2]/Δt mol/L·s |
| 1 | 0.001 | 0.001 | 7 X 10-6 |
| 2 | 0.001 | 0.002 | 1.4 X 10-5 |
| 3 | 0.001 | 0.003 | 2.1 X 10-5 |
| 4 | 0.002 | 0.003 | 8.4 X 10-5 |
| 5 | 0.003 | 0.003 | 1.89 X 10-4 |
Determine the rate law (Answer: rate=k[O2][NO]2 where k = 7000 L2 / mol2 s)
One method for solving that is demonstrated in the following video:
I’ve just given you a quick method that allows you to determine the orders quickly. If you find this confusing, there is a step-by-step method that requires more work but you may find more straightforward. Additionally, it works even when the numbers are more difficult. This step by step method is modeled very well for different problems in Examples 12.4 and 12.5 in OpenStax.
Objective 7 example continued
For the above example, determine the rate when [NO] = 0.0025 mol/L and [O2] = 0.00085 mol/ L
From above, we know the rate law is:
Plugging in the given concentrations,
Yielding an overall rate of 3.72 X 10-5 mol/L⋅s
Objective 7 Practice
Consider the following reaction and its rate law:
Answer the following two questions:
For the following reaction:
Objective 9: Determine whether a rate law for a reaction of form A → products is zero, 1st, or 2nd order given concentration vs. time data. Know what graphs can be used to make this determination, and what quantities are represented but the slope of the resulting line.
Objective 10: Determine the rate constant from the slope and the concentration at a given time from the integrated rate law equation resulting from the plot of first-order data.
Integrated rate laws
Integrated rate laws give reactant concentration as a function of time. They are different from rate laws, which give reaction rate as a function of reactant concentration.
Integrated rate laws are obtained by integrating the rate law. If you have studied calculus, you will know what I mean. If you have not, that’s OK, we will just use the results of the calculus (calculus is not required for this course). Since rate laws are different for reactions with different numbers of reactants and orders, integrated rate laws will differ for the different reactants and orders too.
We are only going to consider integrated rate laws with one reactant in CHEM 152. Examples of these types of reactions are:
Note by “one reactant”, I mean one reactant species, not a coefficient of one. If, in the generic sense, we call this reactant “A”, we can summarize these reactants in the form
A→products
where A is the reactant species and there are one or more product species.
We will consider “A→products” reactions with zero order, first order, and second order rate laws.
First Order Integrated Rate Law – form and graphical representation
For a first order reaction with reactant A, the rate law is:
The integrated rate law is:
where
- k is the rate constant
- t is time
- [A] is the concentration of A at time t
- [A0] is the concentration of A at time=0 (the initial concentration)
This integrated rate law can be expressed graphically in a useful form. The equation is of the form y=mx + b, which is familiar to you as a straight line.
y is ln[A], m is -k, x is t (time), and b is ln[A]0. That means if a reaction is first order, the graph of ln[A] versus time “t” will yield a straight line with a slope of –k and a y intercept of ln[A0]. ![graph of ln [A] vs time ln[A] is plotted on the vertical (y) axis and time is plotted on the horizontal x axis. The graph shows a straight line with a downward slope.](http://fe2.openlcc.net/chem152/files/2020/09/first-order-graph-300x164.png)
Second Order Integrated Rate Law – form and graphical representation
For a second order reaction with a single reactant A, the rate law is:
The integrated rate law is:
where once again
- k is the rate constant
- t is time
- [A] is the concentration of A at time t
- [A0] is the concentration of A at time=0 (the initial concentration).
A graph of ln[A] vs time, which gave a straight line with negative slope for a first order reaction, will not give a straight line for a second order reaction. So we can consider a plot of ln[A] vs time a “test” for first order. However, a look at the second order integrated rate law with the equation of a line shows us that a graph of 1/[A] versus time “t” will yield a straight line with a slope of k and a y intercept of 1/[A0].
![graph of 1/[A] versus time 1/[A] is plotted on the vertical (y) axis and time is plotted on the horizontal x axis. The graph shows a straight line with a upward slope](http://fe2.openlcc.net/chem152/files/2020/09/second-order-integrated-rate-law-300x180.png)
Zero Order Integrated Rate Law – form and graphical representation
For a zero order reaction with reactant A, the rate law is:.
The integrated rate law is:
where
- k is the rate constant
- t is time
- [A] is the concentration of A at time t
- [A0] is the concentration of A at time=0 (the initial concentration)
The graphical test for zero order is a plot of [A] vs. time. If a reaction is zero order, the graph of [A] versus time “t” will yield a straight line with a slope of –k and a y intercept of [A0].![graph of reactant concentration versus time [A] is plotted on the vertical (y) axis and time is plotted on the horizontal x axis. The graph shows a straight line with a downward slope](http://fe2.openlcc.net/chem152/files/2020/09/zero-order-integrated-rate-law-300x164.png)
Objective 9 and 10 Practice
Objective 8: Using the integrated rate law equation for first order reactions only, calculate the rate constant, half-life, time or percent of starting material given any two of the variables.
In our last objective, you were introduced to the integrated rate law for Zero, first, or second order reactions with a single reactant. These integrated rate laws had four variables:
- k is the rate constant
- t is time
- [A] is the concentration of A at time t
- [A0] is the concentration of A at time=0 (the initial concentration)
Given any three of the variables, you should be able to solve for the fourth. In CHEM 152, we will only solve this type of problem for first order reactions. As you saw previously, for a first order reaction with reactant A, the rate law is:.
The integrated rate law is: , where
- k is the rate constant
- t is time
- [A] is the concentration of A at time t
- [A0] is the concentration of A at time=0 (the initial concentration)
There are other forms of this rate law that you might see – these other forms, while they are not set up to give the y=mx+b form of a line, are often more convenient to use when solving these problems.
Using any of the three forms, try solving the practice questions below:
Objective 8 Practice
For the reaction 4 PH3→ P4 + 6 H2, a plot of ln[PH3] vs. time in seconds yields a straight line of slope –0.0347.
Half Life
Half life is defined as the time required for the concentration of a reactant to drop to one half of its initial concentration – we can also think of it as the time it takes for half of the reactant to be consumed. Half life is symbolized by the letter t for time and a subscript of ½, or t1/2.
Consider the following data for the first order reaction A→ products. Notice the starting concentration of A is 1.00 M. After 20 seconds, [A] has fallen to half that value. The half life is 20.0 seconds.
| [A] (mol/L) | Time (sec) |
| 1.0000 | 0.o |
| 0.5000 | 20.0 |
| 0.2500 | 40.0 |
| 0.1250 | 60.0 |
| 0.0625 | 80 |
If we start with the integrated rate law:
At the half life, t = t1/2 and [A] is one half of [A0]. Since [A] is one half of [A0],
Therefore,
The above equation is for first order reaction half life only, as it was derived from the first order integrated rate law. Another feature unique to first order reactions are that successive half lives are equal. What does that mean? If you look at the data above, It takes 20 sec for the molarity of A to drop from 1.0000 to 0.5000, another 20 seconds for the next half life (0.5000 to 0.2500 M), another 20 seconds for the next half life (0.2500 to 0.1250 M), and so on. In reactions with first order rate laws, successive half lives will be equal, and if successive half lives are equal, the reaction will be first order.
In CHEM 152, you are only responsible for numerical calculations involving half life for first order A→products reactions. However, you should understand the concept and definition of half life for reactions of any order.
More Objective 8 Practice
For the reaction 4 PH3→ P4 + 6 H2, the half life is 20.0 seconds. The rate law is first order. Answer the following questions:
Objective 17: Describe the collision model for chemical reactions.
Objective 4: Explain the effect of the physical state of the reactants, frequency, kinetic energy (temperature), catalyst, and orientation of collisions on the reaction rate.
The collision model assumes that reactions occur as a result of collisions between reactant molecules. Once molecules collide they may react or they may not, depending on two factors
- The energy of collision must be sufficient to furnish the minimum necessary energy (activation energy, Ea) for the reaction to occur
- Whether the reacting molecules collide in the proper orientation for new bonds to form. The example for the reaction shown on Figure 12.13 in Open Stax for the reaction CO + O2→CO2 + O.
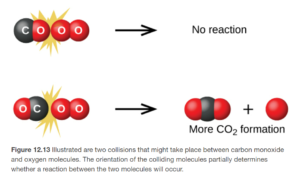
The more collisions that occur, the faster the reaction will progress. As reaction concentrations are increased, there are more reactant molecules in an equivalent volume, and more collisions. This agrees with the mathematics of the rate law, where if concentrations are increased the rate increases.
Increasing temperature also increases rate. As temperature increases, reactant molecules move faster, so they will collide more often (increasing rate). Additionally, when the reactant molecules are moving faster they will collide more energetically, and they are more likely to furnish the activation energy required for the reaction to occur (so the reaction will go faster).
If you look at a rate law mathematically:,
increasing temperature does not increase concentration ([A] or [B]), or order (n or m), but it does increase rate. Therefore, increasing temperature must increase the rate constant k. We will discuss the relationship between temperature and k (the rate constant) later in this topic.
Objective 18: Draw reaction profile diagrams and identify reactants, products, the activated complex, Eact and ΔE.
A reaction profile diagram is a graph showing progress or extent of reaction (sometimes called reaction coordinate) on the x-axis and potential energy on the y-axis. Figure 12.14 in OpenStax shows one of these diagrams. Click the link to open the diagram and refer to it as you read the next few sentences, which will describe it.
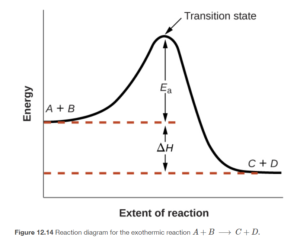
The reaction begins with the reactants A andB, which have a certain potential energy (shown by the height of the curve) at the left. The reaction occurs as you go left to right on the graph, ending on the right with the products C and D. Notice that the product (right) side is lower than energy than the reactant (left) side. This indicates an exothermic reaction, in which the reaction system lowers in energy, releasing th at energy to the surroundings. ΔH (the heat of reaction – see objectives 4 and 5 from this chapter in my CHEM 151 notes for review) is the difference between the beginning energy of the reactants and the ending energy of the products.
As you trace the curve left to right, for the reaction to progress from reactants to products, it must go through what is called a transition state or activated complex. The transition state is a theoretical, high-energy, transient complex between reactant and product. Even though the reaction is exothermic, in order to occur it must have the energy required to “get over the hill” posed by the activated complex. This energy, which on the graph is the difference between the energy of the reactants and the energy of the activated complex, is the activation energy (Ea).
The activation energyis the minimum energy required to initiate a chemical reaction. This energy is supplied by the collision of the reacting molecules. Ea can also be defined as the amount of energy needed to convert reactants into the activated complex (or transition state). The activation energy does affect the reaction rate – the lower the activation energy, the faster the reaction (all else being the same).
Here is a reaction profile diagram for an endothermic reaction:
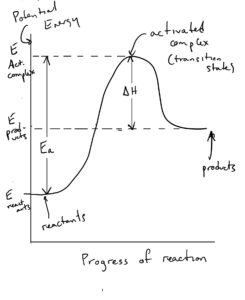
You should know how to draw the reaction profile diagrams for exothermic and endothermic reactions and identify reactants, products, the activated complex, Ea and ΔE.
Objective 22: Explain the temperature dependence of reaction rates.
Objective 23: Define and calculate Eact, the Arrhenius constant or frequency factor (A), rate constant (k), or temperature for a chemical reaction.
Arrhenius’ Law
Arrhenius’ Law shows the relationship between activation energy (Ea) , temperature (T), and the rate constant (k). The energy of collision must be sufficient to furnish necessary energy (activation energy) for the reaction to occur.
Arrhenius’ Law can be written as:
where:
- k = rate constant
- A = Arrhenius Constant or frequency factor
- Ea= Activation Energy
- R = 8.3145 J/mol·K
- T = Kelvin temperature
Let’s look at the equation a little more closely.
Mathematically, as T increases, -Ea/RT will be a smaller negative exponent, making k larger (meaning a faster reaction rate). This matches collision theory, which says that as temperature increases, the velocity of reactant molecules and the kinetic energy of the reactant molecules increases. This means a greater fraction of the collisions are energetic enough to supply the activation energy.
Also, looking at the equation, lower activation energies again make -Ea/RT a smaller negative number, making k larger. This means more collisions at a given temperature will be sufficiently energetic to supply the activation energy.
Overall, low temperature and high activation energy mean will approach zero, meaning no collisions will be energetic enough to supply Ea. High temperature and low activation energy mean
will approach one, meaning every collisions will be energetic enough to supply Ea. Usually, reactions are somewhere between those two extremes.
Solving problems using the Arrhenius Law
One type of problem you can solve using the Arrhenius Law is simple application of the equation– using , given values for all the variables except one, solve for the remaining variable.
More often, though, the data available (via experimentation) is only rate constant (k) and temperature, leaving two unknowns. That means k values must be known at a minimum of two different temperatures (two data points) to solve for Ea and A. This can be done either graphically or with a two data point algebraic form of the equation.
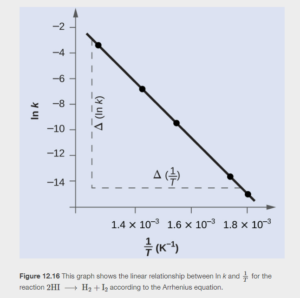
Graphical approach to the Arrhenius Law
The Arrhenius Law can be manipulated algebraically to give this form:
This is of the form y=mx+b, where ln k is y, -Ea/R is the slope m, 1/T is x, and ln A is the y-intercept b.
Therefore, a graph of 1/T on the x-axis and ln k on the y-axis gives a straight line with
The line will slope downward since activation energy is always positive.
Figure 12.16 in OpenStax shows the graph and gives an example of its use.
Objective 24: Using two sets of temperature and rate constants, calculate any of the following variables: the activation energy, the temperature, or rate constant
Two data point algebraic form of the Arrhenius Law
Using the graph of ln k vs 1/T discussed in the previous objective,
for two data points (k1 is the rate constant at temperature T1 and k2 is the rate constant at temperature T2 )
This can be rearranged to yield:
which is the two data point form of the Arrhenius Equation. It requires rate constant (k) values at 2 different temperatures. The T must be in Kelvin. Also, Ea and R must contain the same energy units. If you use R=8.3145 J/mol⋅K, the Ea will be in J or J/mol. This is a common source or error as activation energies are often quoted in joules. Make sure either you convert Ea to J or R to kJ/mol⋅K.
Given all but one variable, you should be able to solve the 2 data point form. Here, it is use to determine the activation energy of the reaction.
Objective 24 Practice
For the reaction 2 NOCl (g)→ 2 NO(g)+Cl2(g), the rate constant at 300.0 K is 2.6 X 10-8. The rate constant at 400.0 K is 4.9 X 10-4 . Calculate the activation energy to two significant figures.
Objective 12: Write the rate law for elementary reactions/steps.
Objective 13: Discuss the relationship between the reaction mechanism and the rate law.
Objective 14: Determine the molecularity of each elementary reaction, rate determining step and the overall balanced equation given the mechanism for a reaction.
Objective 15: Given the mechanism for a reaction write the rate law.
When you see a balanced chemical equation, all that is written is the reactants and the products. There is no detail of how the chemical change from reactants take place. It is similar to only seeing the beginning and ending scenes in a movie, missing the entire plot in between that shows how it progresses to the end. A reaction mechanism provides step by step details of the actual changes that occur during a reaction. Most reactions occur in a series of steps involving 1, 2, or at most 3 molecules. There can be one or two steps, or more complicated mechanisms can have many more. These individual steps making up the reaction mechanism are called elementary reactions or elementary steps or elementary processes.
Here is an example of a reaction mechanism for the reaction:
Adding together the 2 steps, and cancelling an NO2 and NO3 from each side since they appear on both sides, yields the overall (or net) reaction:
The series of elementary steps that makes up a mechanism must satisfy two requirements:
- The sum of the elementary steps must give the overall balanced (net) equation for the reaction (we just saw that in the reaction above)
- The mechanism must agree with experimentally determined rate law (we will see that later)
Intermediates in reaction mechanisms
An intermediate is a product in an earlier step and a reactant in a later step. Intermediates do not show up in the overall reaction and do not appear in the rate law.
Intermediate Example
For the mechanism we looked at above:
The intermediate is NO3. It is produced in Step 1 and is a reactant in Step 2. It does not appear in the overall reaction
Elementary Steps, Rate Laws, and Molecularity
The molecularity of a reaction refers to the number of separate molecules or atoms that participate as reactants in an elementary step. Since the reactants in an elementary step actually collide , you can write a rate law directly for an elementary step. You cannot write a rate law directly for an overall (net) reaction.
Let’s continue our look at the mechanism we have been examining.
Overall (or net) reaction:
You may recall from earlier that we cannot write a rate law knowing the orders for the overall reaction without experimental data. This is because the reactants in a net reaction may not actually collide. But the elementary steps in a reaction mechanism actually do collide, so we can write a rate law directly from the elementary steps, using the coefficients as powers:
For , the rate law is:
For , the rate law is:
Unimolecular Elementary Reactions
Elementary steps with a single reactant molecule have a molecularity of 1. They are called unimolecular. They have the form A→products and a rate law of .
Unimolecular elementary steps will be first order.
Bimolecular Elementary Reactions
Elementary steps with two reactant atoms or molecules have a molecularity of 2. They are called bimolecular. They have the forms A+B→products or 2A→products and a rate laws of:
or
Bimolecular elementary steps will be second order.
Termolecular Elementary Reactions
Termolecular elementary steps are very rare as the probability of three independently moving molecules or atoms colliding simultaneously is very low. But they do sometimes occur. Their molecularity and order are 3.
OpenStax Section 12.6 has more examples of unimolecular, bimolecular, and termolecular elementary steps.
Rate Determining Step in a Mechanism
The rate determining step is the slowest elementary step of a mechanism. When a reaction occurs with more than one elementary step, usually one of the elementary steps is much slower than the others. It is the step with the largest activation energy. We can assume that the rate law of the rate determining step is the rate law of the overall reaction, as it is so much slower than the other steps that it dominates and determines the rate.
Rate Determining Step Example
Let’s look one last time at our example mechanism and reaction (with one additional piece of given information- now I’m going to tell you which step is the slow, or rate-determining, step):
For the following overall reaction:
The proposed mechanism is
The rate law for the first, slow step is:
The rate law for the second, fast step is:
since the first step is the rate determining step, the overall rate law is:.
The way we could test for evidence on whether this proposed mechanism is the actual mechanism is to run experiments with the overall reaction and see if it is actually second order in NO2.
Objective 15 practice
The following mechanism has been proposed for the gas phase reaction of hydrogen and iodine monochloride:
Objective 16: Given a mechanism in which the rate determining step is not the first step, write the rate law.
Consider the following three step mechanism for a reaction:
fast
slow
fast
- What is the overall reaction?
- Identify the intermediates in the reaction.
- What is the predicted rate law?
The problem is solved in the video below
Objective 19: Describe the effect of a catalyst on the energy profile diagram of a reaction.
Objective 20: Explain how a catalyst works and how it fits into the collision model for chemical reactions.
Objective 21: Identify the catalyst and the intermediate given the mechanism for a reaction.
Catalysts are substances that increase the rate of a reaction by decreasing the activation energy of the reaction. They are often described as speeding up a reaction without being consumed. They do this by changing the mechanism by which the process occurs, and providing a new mechanism with a lower activation energy. The energy of the reactants, the energy of the products, and the ΔH are unchanged.
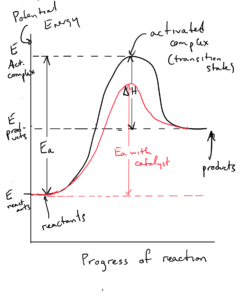
Catalysts can show up in mechanism as being consumed in an earlier step and reappearing in a later step.
A heterogeneous catalyst is in different phase than reacting molecules (usually a solid). A homogeneous catalyst exists in the same phase as the reacting molecules. In biology, Enzymes are highly specific catalysts. They can increase rate by a factor of 106 to 1012.
Objective 21 practice
This concludes our look at chemical kinetics. As you will see, aspects of kinetics will come up in our next topic (Nuclear Chemistry), as well as briefly in its connection with Thermodynamics in Unit 3.